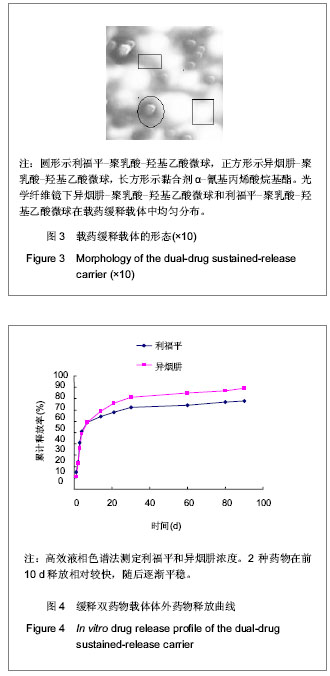
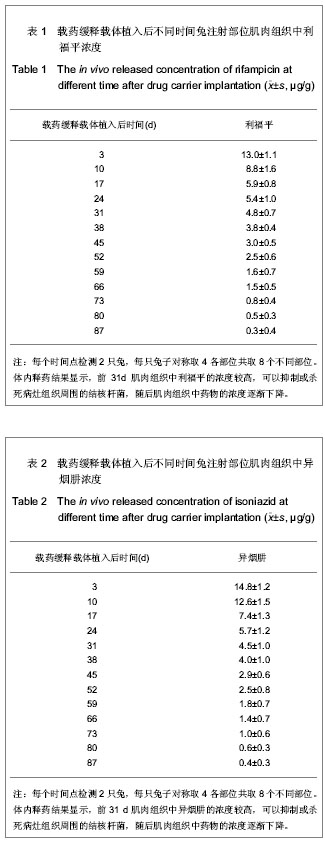
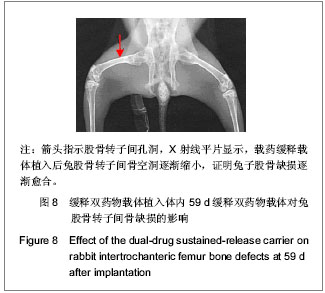
| [1] Sundararaj GD, Behera S, Ravi V, et al. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85(1):100-106.[2] 施建党,王自立,马小民.病灶清除植骨内固定治疗相邻多椎体脊柱结核[J].中国脊柱脊髓杂志,2010,20(2):98-102.[3] Ozdemir HM, Us AK, O?ün T. The role of anterior spinal instrumentation and allograft fibula for the treatment of pott disease. Spine (Phila Pa 1976). 2003;28(5):474-479.[4] 瞿东滨,金大地.正确认识脊柱结核病灶清除术[J].中国脊柱脊髓杂志,2008,18(8):565-567.[5] 第四届全国脊柱及骨关节结核病专题研讨会纪要[J].中国脊柱脊髓杂志,2010,20(11):965-968.[6] Kumar G, Sharma S, Shafiq N, et al. Pharmacokinetics and tissue distribution studies of orally administered nanoparticles encapsulated ethionamide used as potential drug delivery system in management of multi-drug resistant tuberculosis. Drug Deliv. 2011;18(1):65-73. [7] 郭虹,李环,李淑芬,等.抗结核药所致肝损害的发生机制及影响因素[J].北华大学学报(自然科学版),2006,7(2):159-161.[8] 毕经瑞,苏华田,程莹,等.抗结核药物致肝损127例分析[J].中国防痨杂志,2002,24(4):233-235.[9] 刘会民,张洁,张永进.我院异烟肼血药浓度监测结果分析[J].天津药学2011,23(6):27-28.[10] 田洪英,王洪星,姚明.抗结核药血药浓度监测及临床疗效与安全性分析[J].中国药房,2012,23(26):2485-2487.[11] 王自立.脊柱结核的病灶清除与融合固定问题[J].中国脊柱脊髓杂志,2006,16(12):888-889.[12] Ge Z, Wang Z, Wei M. Measurement of the concentration of three antituberculosis drugs in the focus of spinal tuberculosis. Eur Spine J. 2008;17(11):1482-1487.[13] 鲍玉成,张文龙,王勇,等.长效缓释双药物人工骨的制备及释放特性[J].中国组织工程研究,2012,16(38):7126-7130.[14] 刘江涛,王永清,夏侃,等.异烟肼聚乳酸缓释体的制备及体内外释药特性[J].中国脊柱脊髓杂志,2008,18(4):290-293.[15] Arshady R. Microspheres and microcapsules, a survey of manufacturing techniques: Part III: Solvent evaporation. Polym Eng Sci. 1990;30(15):915-924.[16] 叶向阳,孙湘,贾会文,等.利福平聚乳酸-聚羟基乙酸缓释微球的制备及特性[J].中国组织工程研究与临床康复,2011;15(51): 9608-9612.[17] 叶向阳,甄平,李晓飞,等.新型抗结核多孔磷酸钙骨水泥缓释载体的制备与性能研究[J].中国矫形外科杂志2010,18(23):1981-1986.[18] 应小樟,徐华梓,彭磊,等.利福平硫酸钙植入剂的研制与相关性能检测[J].温州医学院学报,2007,1(37):50-55.[19] 罗培培,左奕,孙斌,等.载异烟肼聚己内酯微球的制备及其性质研究[J].功能材料,2011,42(8):1410-1414.[20] Loo SC, Tan ZY, Chow YJ, et al. Drug release from irradiated PLGA and PLLA multi-layered films. J Pharm Sci. 2010;99(7): 3060-3071. [21] Parveen S, Sahoo SK. Polymeric nanoparticles for cancer therapy. J Drug Target. 2008;16(2):108-123.[22] 张宏其,高琪乐,郭虎兵,等.人同种异体骨载异烟肼-壳聚糖缓释微球的制备和体内外释药特性[J].中国组织工程研究,2012, 16(3):479-483.[23] World Health Organization. Global tuberculosis report 2012 [EB/OL]. http://www.who.int/tb/publications/global_report/en/[24] 戈朝晖,王自立,魏敏吉.脊柱结核病灶中抗痨药物浓度的测定[J].中华骨科杂志,2005,25(2):97-101.[25] Pawar UM, Kundnani V, Agashe V, et al. Multidrug-resistant tuberculosis of the spine--is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine (Phila Pa 1976). 2009;34(22):E806-810. [26] 肖文德,姬广林,高辉.脊柱结核的诊断与外科治疗进展[J].中国矫形外科杂志,2008,16(5):359-361.[27] 施建党,王自立.脊柱结核术后未愈及术后复发的原因探讨[J].中国矫形外科杂志,2005,13(15):1184-1186.[28] 付裕,霍洪军,肖宇龙,等.强化抗结核药物联合手术治疗胸腰椎结核[J].中国修复重建外科杂志,2009,23(12):1427-1430.[29] 杨君礼,袁牧,许刚,等.利福平粉剂在脊柱结核病灶清除术中的应用观察[J].颈腰痛杂志,2005,26(4):270-273.[30] Dutt M, Khuller GK. Chemotherapy of Mycobacterium tuberculosis infections in mice with a combination of isoniazid and rifampicin entrapped in Poly (DL-lactide-co-glycolide) microparticles. J Antimicrob Chemother. 2001;47(6):829-835.[31] 张厚安,李敏,唐思文.骨组织工程用细胞支架生物材料的研究进展[J].机械工程材料,2007,31(12):4-7.[32] 龚明明,谭丽丽,杨柯.骨组织工程支架材料及其力学性能[J].材料导报,2007,21(10):43-46. |


.jpg)