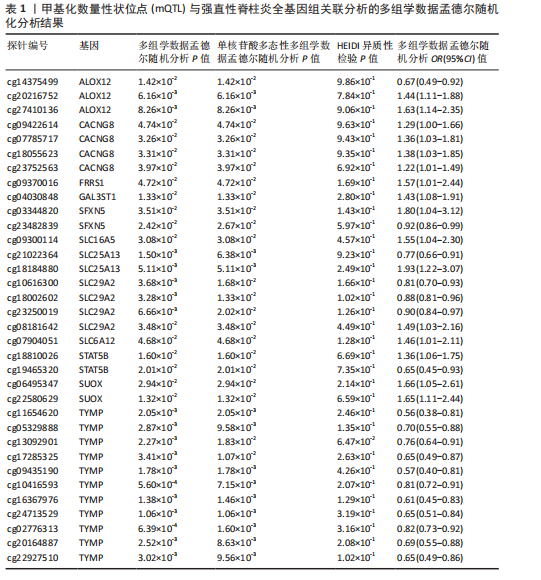
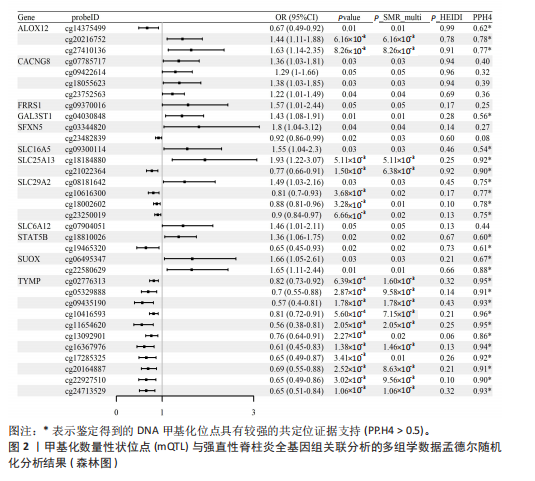
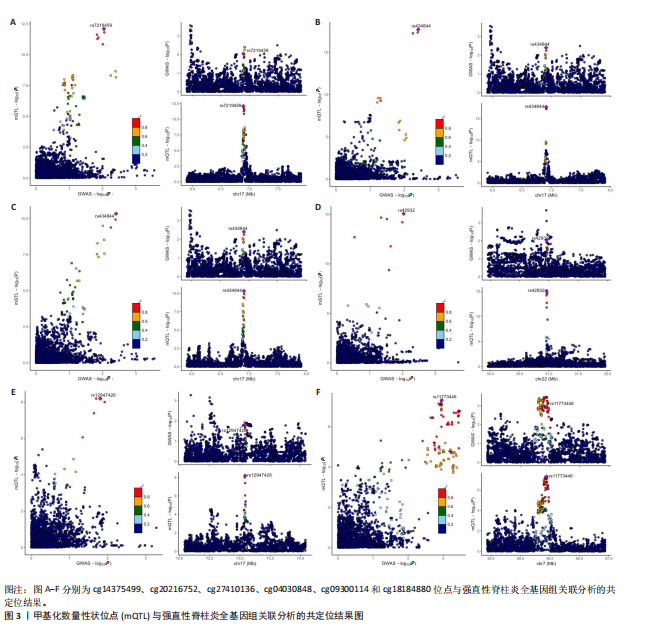
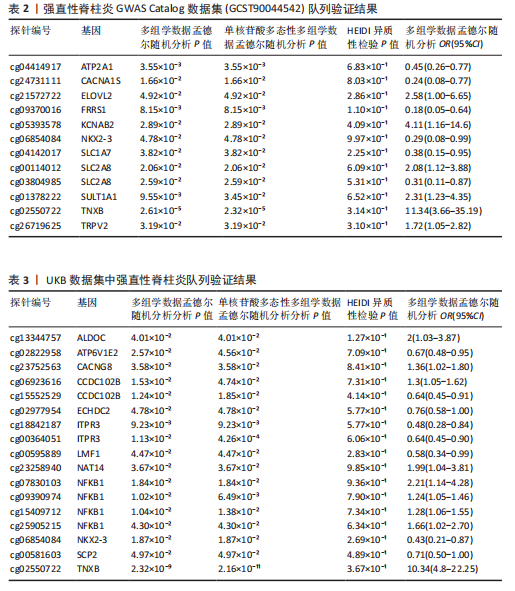
[1] TSVETKOV P, COY S, PETROVA B, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375(6586):1254-1261.
[2] BROWN MA, KENNA T, WORDSWORTH BP. Genetics of ankylosing spondylitis--insights into pathogenesis. Nat Rev Rheumatol. 2016;12(2):81-91.
[3] SOLIER S, MÜLLER S, CAÑEQUE T, et al. A druggable copper-signalling pathway that drives inflammation. Nature. 2023; 617(7960):386-394.
[4] AIGINGER P, KOLARZ G, WILLVONSEDER R. Copper in ankylosing spondylitis and rheumatoid arthritis. Scand J Rheumatol. 1978;7(2):75-78.
[5] COBINE PA, BRADY DC. Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. 2022; 82(10):1786-1787.
[6] COBINE PA, MOORE SA, LEARY SC. Getting out what you put in: Copper in mitochondria and its impacts on human disease. Biochim Biophys Acta Mol Cell Res. 2021;1868(1):118867.
[7] 朱赛雅,刘晶,余晨.线粒体铜稳态失衡与纤维化疾病的研究进展[J].生理学报, 2024,76(4):597-604.
[8] ZHOU Y, ZHANG L. The interplay between copper metabolism and microbes: in perspective of host copper-dependent ATPases ATP7A/B. Front Cell Infect Microbiol. 2023;13:1267931.
[9] ZHANG P, CHEN H, ZHANG Y, et al. Dry and wet experiments reveal diagnostic clustering and immune landscapes of cuproptosis patterns in patients with ankylosing spondylitis. Int Immunopharmacol. 2024; 127:111326.
[10] KARDOS J, HÉJA L, SIMON Á, et al. Copper signalling: causes and consequences. Cell Commun Signal. 2018;16(1):71.
[11] JAYSON MI, DAVIS P, WHICHER JT, et al. Serum copper and caeruloplasmin in ankylosing spondylitis, systemic sclerosis, and morphea. Ann Rheum Dis. 1975;35(5): 443-445.
[12] FAN J, LIU Q, CHEN T, et al. Identification of cuproptosis-related genes related to the progression of ankylosing spondylitis by integrated bioinformatics analysis. Medicine (Baltimore). 2024;103(35):e38313.
[13] WU Y, ZENG J, ZHANG F, et al. Integrative analysis of omics summary data reveals putative mechanisms underlying complex traits. Nat Commun. 2018;9(1):918.
[14] VÕSA U, CLARINGBOULD A, WESTRA HJ, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. 2021; 53(9):1300-1310.
[15] SUN BB, CHIOU J, TRAYLOR M, et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature. 2023; 622(7982):329-338.
[16] PAIRO-CASTINEIRA E, RAWLIK K, BRETHERICK AD, et al. GWAS and meta-analysis identifies 49 genetic variants underlying critical COVID-19. Nature. 2023; 617(7962):764-768.
[17] ZHU Z, ZHANG F, HU H, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481-487.
[18] ORTEGA A, TARAZÓN E, ROSELLÓ-LLETÍ E, et al. Patients with Dilated Cardiomyopathy and Sustained Monomorphic Ventricular Tachycardia Show Up-Regulation of KCNN3 and KCNJ2 Genes and CACNG8-Linked Left Ventricular Dysfunction. PLoS One. 2015; 10(12):e0145518.
[19] BAI WJ, LUO XG, JIN BH, et al. Deficiency of transmembrane AMPA receptor regulatory protein γ-8 leads to attention-deficit hyperactivity disorder-like behavior in mice. Zool Res. 2022;43(5):851-870.
[20] WU X, GAO L, ZhOU K, et al. Deposition and transport of trace mineral elements were affected by stocking density in fattening pigs. J Trace Elem Med Biol. 2018;50:566-571.
[21] WANG H, LI J, ZI X, et al. Comprehensive analysis of cuproptosis-related genes on bladder cancer prognosis, tumor microenvironment invasion, and drug sensitivity. Front Oncol. 2023;13:1116305.
[22] LIU H, LIU L, LIU Q, et al. LncRNA HOXD-AS1 affects proliferation and apoptosis of cervical cancer cells by promoting FRRS1 expression via transcription factor ELF1. Cell Cycle. 2022;21(4):416-421.
[23] GUERRA LN, SUAREZ C, SOTO D, et al. GAL3ST2 from mammary gland epithelial cells affects differentiation of 3T3-L1 preadipocytes. Clin Transl Oncol. 2015; 17(7):511-520.
[24] ROBINSON CM, POON BPK, KANO Y, et al. A Hypoxia-Inducible HIF1-GAL3ST1-Sulfatide Axis Enhances ccRCC Immune Evasion via Increased Tumor Cell-Platelet Binding [published correction appears in Mol Cancer Res. 2021;19(4):739.
[25] 翁勰,肖芦山,邹雪晶,等.GAL3ST1对肝细胞癌肿瘤进展的影响及机制研究[J].临床肿瘤学杂志,2022,27(6):488-494.
[26] MENG Q, HU X, ZHAO X, et al. A circular network of coregulated sphingolipids dictates lung cancer growth and progression. EBioMedicine. 2021;66: 103301.
[27] CHEN L, ELIZALDE M, DUBOIS LJ, et al. GAL3ST1 Deficiency Reduces Epithelial-Mesenchymal Transition and Tumorigenic Capacity in a Cholangiocarcinoma Cell Line. Int J Mol Sci. 2024;25(13):7279.
[28] NAITO Y, AKIBA J, KINJO Y, et al. Predictive and Prognostic Value of SUOX Expression in Pancreatic Ductal Adenocarcinoma. Anticancer Res. 2022;42(8):4145-4151.
[29] TOKISAWA S, KONDO R, NAKAYAMA M, et al. Clinicopathological significance of sulfite oxidase expression in surgically resected lung adenocarcinoma. Med Mol Morphol. 2025;58(2):106-113.
[30] JACKSON TD, HOCK DH, FUJIHARA KM, et al. The TIM22 complex mediates the import of sideroflexins and is required for efficient mitochondrial one-carbon metabolism. Mol Biol Cell. 2021;32(6):475-491.
[31] ZHANG H, MENG L, LIU Y, et al. Sfxn5 Regulation of Actin Polymerization for Neutrophil Spreading Depends on a Citrate-Cholesterol-PI(4,5)P2 Pathway. J Immunol. 2023;211(3):462-473.
[32] JIN Q, REN F, SONG P. Innovate therapeutic targets for autoimmune diseases: insights from proteome-wide mendelian randomization and Bayesian colocalization. Autoimmunity. 2024;57(1):2330392.
[33] TAO Y, HUA G, MIN S, et al. Verification of biological markers of subacute cutaneous lupus erythematosus via TMT labelling proteomics combined with transcriptome data. Ann Med. 2025;57(1):2500696.
[34] PALADHI A, DARIPA S, MONDAL I, et al. Targeting thymidine phosphorylase alleviates resistance to dendritic cell immunotherapy in colorectal cancer and promotes antitumor immunity. Front Immunol. 2022;13:988071.
[35] YOU W, LIN Y, LIU M, et al. Investigating potential novel therapeutic targets and biomarkers for ankylosing spondylitis using plasma protein screening. Front Immunol. 2024;15:1406041.
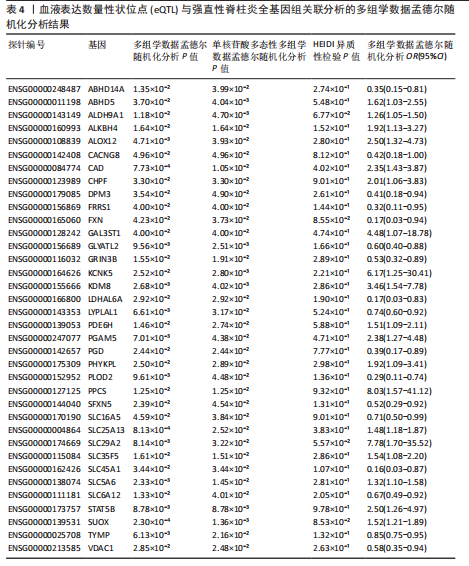
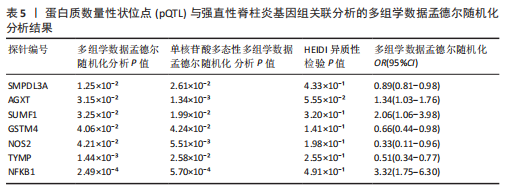
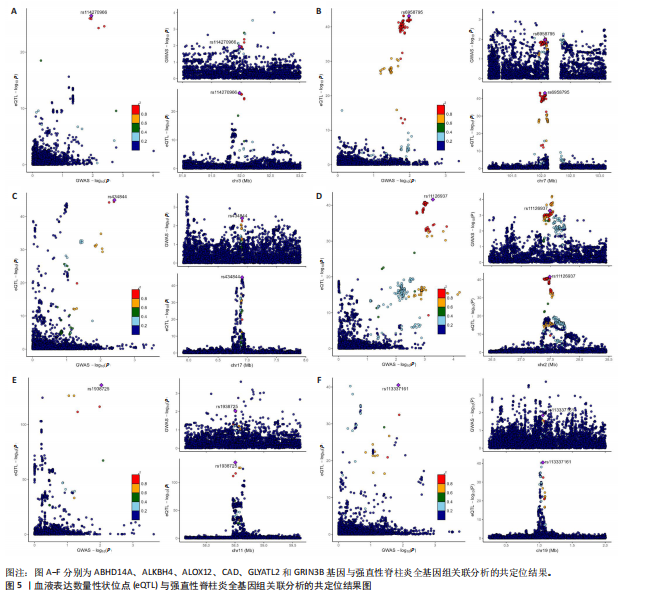
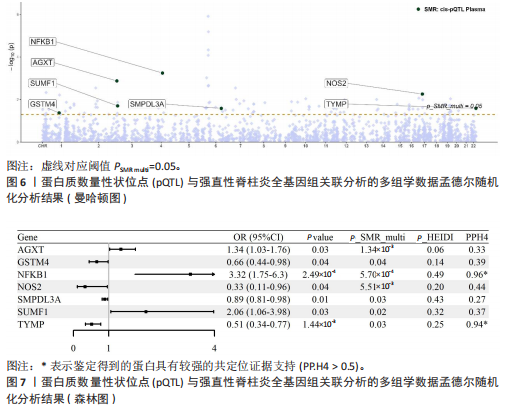
|