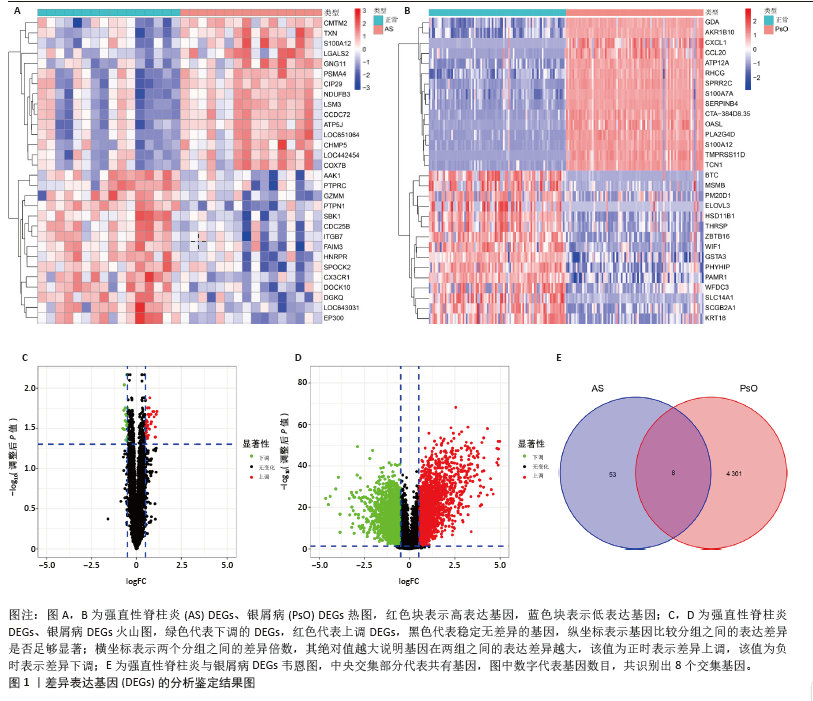
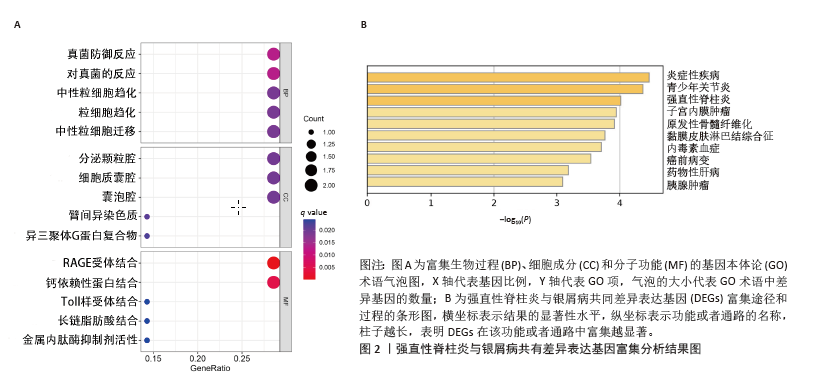
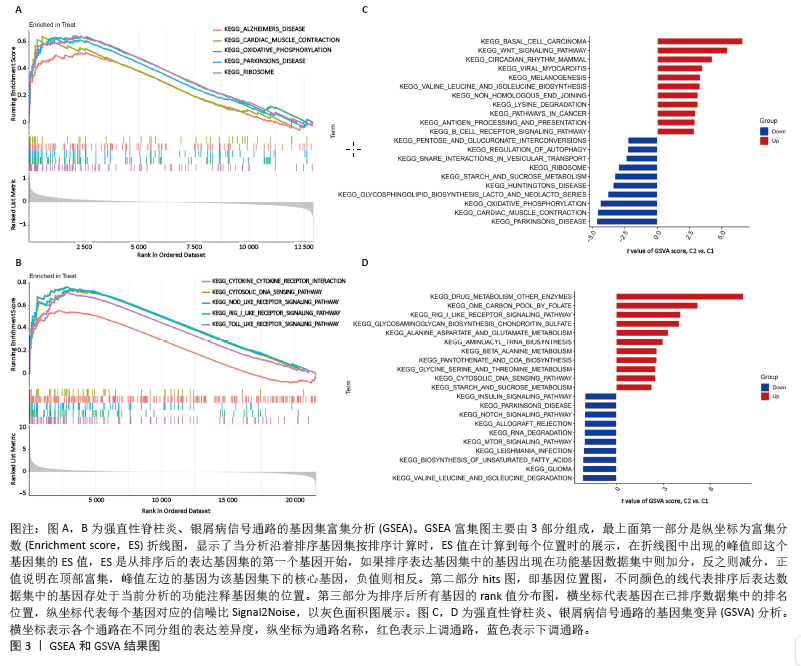
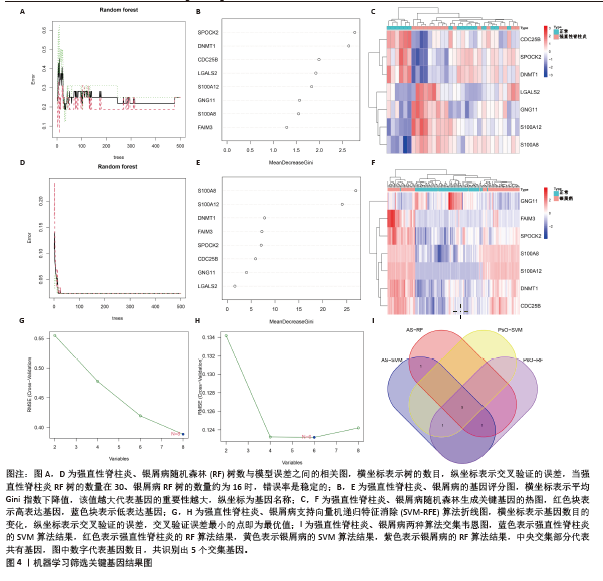
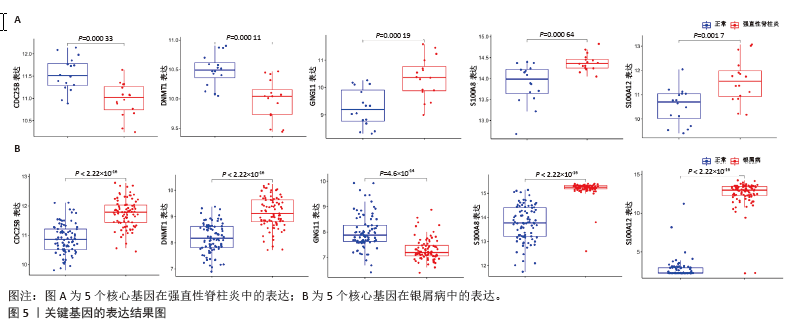
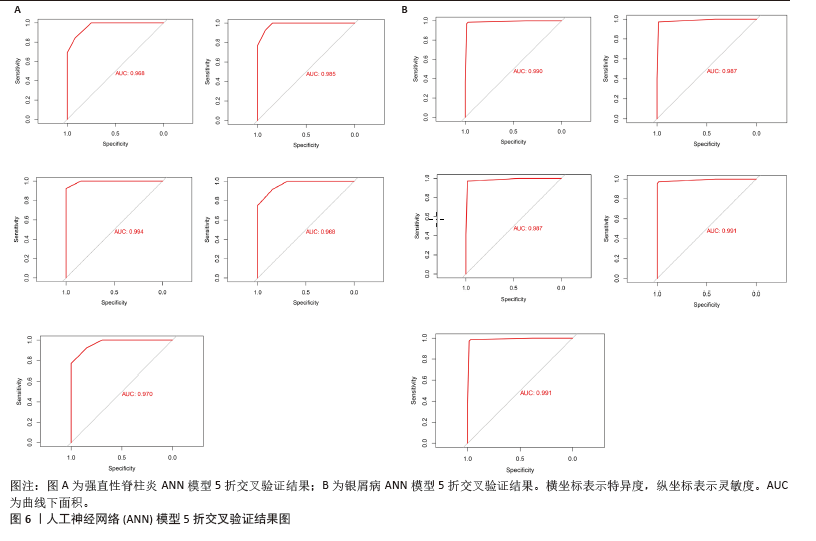
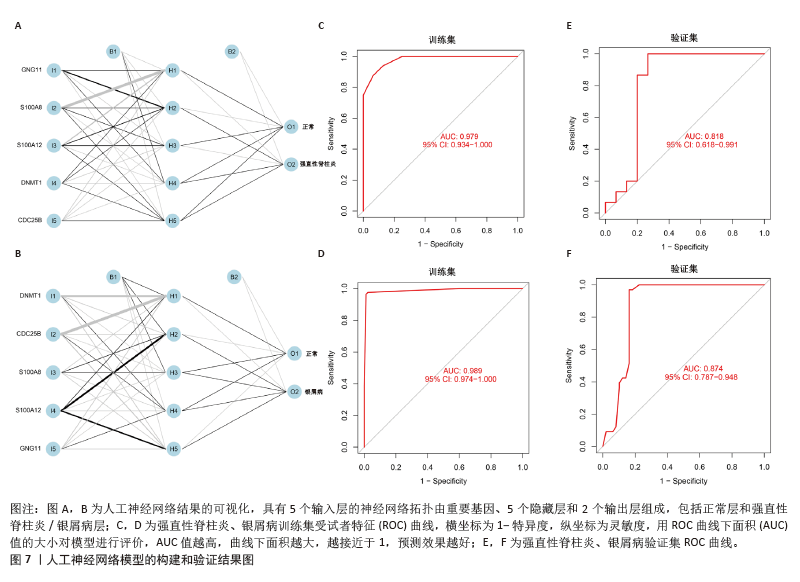
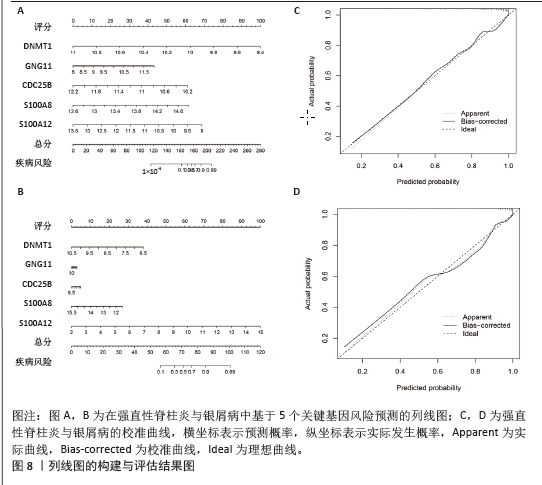
[1] TAUROG JD, CHHABRA A, COLBERT RA. Ankylosing spondylitis and axial spondyloarthritis. New Engl J Med. 2016; 374(26):2563-2574.
[2] WARD MM, DEODHAR A, GENSLER LS, et al. 2019 update of the american college of rheumatology/spondylitis association of america/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arth Rheum. 2019;71(10):1285-1299.
[3] RANGANATHAN V, GRACEY E, BROWN MA, et al. Pathogenesis of ankylosing spondylitis—recent advances and future directions. Nat Rev Rheumatol. 2017;13(6):359-367.
[4] CHEN Y, WU Y, YANG H, et al. DNA Methylation and mRNA expression of B7-H3 gene in ankylosing spondylitis: a case-control study. Immunol Invest. 2022;51(7):2025-2034.
[5] CHOUDHARY S, KHAN NS, VERMA R, et al. Exploring the molecular underpinning of psoriasis and its associated comorbidities through network approach: cross talks of genes and pathways. 3 Biotech. 2023; 13(5):130.
[6] WANG T, XIA Y, ZHANG X, et al. Short-term effects of air pollutants on outpatients with psoriasis in a Chinese city with a subtropical monsoon climate. Front Public Health. 2022; 10:1071263.
[7] GUO J, ZHANG H, LIN W, et al. Signaling pathways and targeted therapies for psoriasis. Signal Transduct Target Ther. 2023;8(1):437.
[8] GRIFFITHS CEM, ARMSTRONG AW, GUDJONSSON JE, et al. Psoriasis. Lancet. 2021; 397(10281):1301-1315.
[9] CHISĂLĂU BA, CRÎNGUȘ LI, VREJU FA, et al. New insights into IL‑17/IL‑23 signaling in ankylosing spondylitis. Exp Ther Med. 2020;20(4): 3493-3497.
[10] GEORGESCU SR, TAMPA M, CARUNTU C, et al. Advances in understanding the immunological pathways in psoriasis. Int J Mol Sci. 2019;20(3): 739.
[11] INGRASCIOTTA Y, SPINI A, L’ABBATE L, et al.
Comparing clinical trial population representativeness to real-world users of 17 biologics approved for immune-mediated inflammatory diseases: an external validity analysis of 66,639 biologic users from the Italian VALORE project. Pharmacol Res. 2024; 200:107074.
[12] BODUR H, ATAMAN Ş, BUĞDAYCI DS, et al. Description of the registry of patients with ankylosing spondylitis in Turkey: TRASD-IP. Rheumatol Int. 2012;32(1):169-176.
[13] STOLWIJK C, VAN TUBERGEN A, CASTILLO-ORTIZ JD, et al. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(1):65-73.
[14] GOTTLIEB AB, DEODHAR A, MCINNES IB, et al. Long-term safety of secukinumab over five years in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis and ankylosing spondylitis: update on integrated pooled clinical trial and post-marketing surveillance data. Acta Derm Venereol. 2022; 102:adv00698.
[15] MAN S, JI X, HU L, et al. Characteristics associated with the occurrence and development of acute anterior uveitis, inflammatory bowel disease, and psoriasis in patients with ankylosing spondylitis: data from the Chinese ankylosing spondylitis prospective imaging cohort. Rheumatol Ther. 2021;8(1):555-571.
[16] CECCARELLI M, VENANZI RULLO E, BERRETTA M, et al. New generation biologics for the treatment of psoriasis and psoriatic arthritis. State of the art and considerations about the risk of infection. Dermatol Ther. 2021;34(1):e14660.
[17] XU W D, LI R, HUANG A F. Role of TL1A in inflammatory autoimmune diseases: a comprehensive review. Front Immunol. 2022; 13:891328.
[18] JUNG SM, KIM WU. Targeted immunotherapy for autoimmune disease. Immune Netw. 2022; 22(1):e9.
[19] ATHANASSIOU L, KOSTOGLOU-ATHANASSIOU I, KOUTSILIERIS M, et al. Vitamin D and autoimmune rheumatic diseases. Biomolecules. 2023;13(4):709.
[20] KRUEGER JG, WHARTON JR KA, SCHLITT T, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750-763.
[21] BRIDGEWOOD C, NEWTON D, BRAGAZZI N, et al. Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis. Semin Immunol. 2021;58:101520.
[22] TSUKAZAKI H, KAITO T. The role of the IL-23/IL-17 pathway in the pathogenesis of spondyloarthritis. Int J Mol Sci. 2020; 21(17):6401.
[23] CHANDRASEKHARAN UM, KAUR R, HARVEY JE, et al. TNFR2 depletion reduces psoriatic inflammation in mice by downregulating specific dendritic cell populations in lymph nodes and inhibiting IL-23/IL-17 pathways. J Invest Dermatol. 2022;142(8):2159-2172.e9.
[24] RAMIRO S, NIKIPHOROU E, SEPRIANO A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19-34.
[25] TORRES T, PUIG L, VENDER R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567-579.
[26] BERGBOER JGM, OOSTVEEN AM, DE JAGER MEA, et al. Paediatric‐onset psoriasis is associated with ERAP1 and IL23R loci, LCE3C_LCE3B deletion and HLA‐C* 06. Br J Dermatol. 2012;167(4):922-925.
[27] ROBERTS AR, APPLETON LH, CORTES A, et al. ERAP1 association with ankylosing spondylitis is attributable to common genotypes rather than rare haplotype combinations. Proc Natl Acad Sci U S A. 2017;114(3):558-561.
[28] MOHAN KN. DNMT1: catalytic and non-catalytic roles in different biological processes. Epigenomics. 2022;14(10):629-643.
[29] HAY AD, KESSLER NJ, GEBERT D, et al. Epigenetic inheritance is unfaithful at intermediately methylated CpG sites. Nat Commun. 2023;14(1):5336.
[30] HU Y, XU B, HE J, et al. Hypermethylation of Smad7 in CD4+ T cells is associated with the disease activity of rheumatoid arthritis. Front Immunol. 2023;14:1104881.
[31] YAN L, GENG Q, CAO Z, et al. Insights into DNMT1 and programmed cell death in diseases. Biomed Pharmacother. 2023;168: 115753.
[32] REN Y. Regulatory mechanism and biological function of UHRF1–DNMT1-mediated DNA methylation. Funct Integr Genomics. 2022;22(6):1113-1126.
[33] GARSHASBI M, MAHMOUDI M, RAZMARA E, et al. Identification of RELN variant p.(Ser2486Gly) in an Iranian family with ankylosing spondylitis; the first association of RELN and AS. Eur J Hum Genet. 2020;28(6):754-762.
[34] ALFARDAN AS, NADEEM A, AHMAD SF, et al. DNMT inhibitor, 5-aza-2′-deoxycytidine mitigates di (2-ethylhexyl) phthalate-induced aggravation of psoriasiform inflammation in mice via reduction in global DNA methylation in dermal and peripheral compartments. Int Immunopharmacol. 2024;137:112503.
[35] JIANG MM, ZHAO F, LOU TT. Assessment of significant pathway signaling and prognostic value of GNG11 in ovarian serous cystadenocarcinoma. Int J Gen Med. 2021;14:2329-2341.
[36] MENG G, DUAN H, JIA J, et al. Alfalfa xenomiR-162 targets G protein subunit gamma 11 to regulate milk protein synthesis in bovine mammary epithelial cells. Anim Biosci. 2024;37(3):509-521.
[37] ZHANG Y, ZHANG C, CHEN W, et al. The landscape of allelic expression and DNA methylation at the bovine SGCE/PEG10 locus. Anim Genet. 2024;55(3):452-456.
[38] FANG F, PAN J, XU L, et al. Identification of potential transcriptomic markers in developing ankylosing spondylitis: a meta‐analysis of gene expression profiles. Biomed Res Int. 2015; 2015(1):826316.
[39] WROŃSKI A, JAROCKA-KARPOWICZ I, STASIEWICZ A, et al. Phytocannabinoids in the pharmacotherapy of psoriasis. Molecules. 2023;28(3):1192.
[40] CÁNOVAS PM. Survivin mediates mitotic onset in hela cells through activation of the Cdk1-Cdc25B axis. Res Sq. 2024;28: rs3949429.
[41] FERENCOVA I, VASKOVICOVA M, DRUTOVIC D, et al. CDC25B is required for the metaphase I-metaphase II transition in mouse oocytes. J Cell Sci. 2022;135(6):jcs252924.
[42] LI HX, YANG WY, LI LP, et al. Molecular dynamics study of CDC25BR492L mutant causing the activity decrease of CDC25B. J Mol Graph Model. 2021;109:108030.
[43] GOETZKE CC, EBSTEIN F, KALLINICH T. Role of proteasomes in inflammation. J Clin Med. 2021;10(8):1783.
[44] LI L P, LI H X, ZHOU H, et al. Exploring the mechanism of C473D mutation on CDC25B causing weak binding affinity with CDK2/CyclinA by molecular dynamics study. J Biomol Struct Dyn. 2023;41(22):12552-12564.
[45] JARLBORG M, COURVOISIER DS, LAMACCHIA C, et al. Serum calprotectin: a promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res Ther. 2020; 22(1):105.
[46] PETIT RG, CANO A, ORTIZ A, et al. Psoriasis: from pathogenesis to pharmacological and nano-technological-based therapeutics. Int J Mol Sci. 2021;22(9):4983.
[47] XIA P, JI X, YAN L, et al. Roles of S100A8, S100A9 and S100A12 in infection, inflammation and immunity. Immunology. 2024;171(3):365-376.
[48] CERÓN JJ, ORTÍN-BUSTILLO A, LÓPEZ-MARTÍNEZ MJ, et al. S-100 proteins: basics and applications as biomarkers in animals with special focus on calgranulins (S100A8, A9, and A12). Biology. 2023;12(6):881.
[49] SPRENKELER EGG, ZANDSTRA J, VAN KLEEF ND, et al. S100A8/A9 is a marker for the release of neutrophil extracellular traps and induces neutrophil activation. Cells. 2022;11(2):236.
[50] VON WULFFEN M, LUEHRMANN V, ROBECK S, et al. S100A8/A9-alarmin promotes local myeloid-derived suppressor cell activation restricting severe autoimmune arthritis. Cell Rep. 2023;42(8):113006.
[51] BORSKY P, FIALA Z, ANDRYS C, et al. Alarmins HMGB1, IL‐33, S100A7, and S100A12 in psoriasis vulgaris. Mediators Inflamm. 2020; 2020(1):8465083.
[52] SINGH P, ALI SA. Multifunctional role of S100 protein family in the immune system: an update. Cells. 2022;11(15):2274.
[53] ABDI W, ROMASCO A, ALKURDI D, et al. An overview of S100 proteins and their functions in skin homeostasis, interface dermatitis conditions and other skin pathologies. Exp Dermatol. 2024;33(8):e15158.
[54] SREEJIT G, FLYNN MC, PATIL M, et al. S100 family proteins in inflammation and beyond. Adv Clin Chem. 2020;98:173-231.
[55] LEŚNIAK W, FILIPEK A. S100 Proteins—intracellular and extracellular function in norm and pathology. Biomolecules. 2024;14(4):432.
[56] SAGONAS I, ILIOPOULOS G, Baraliakos X, et al. Anti-TNF-α induced paradoxical psoriasis in patients with ankylosing spondylitis: a systematic review. Clin Exp Rheumatol. 2024; 42(1):178-184.
[57] PAN S, WU S, WEI Y, et al. Exploring the causal relationship between inflammatory cytokines and inflammatory arthritis: a Mendelian randomization study. Cytokine. 2024;173:156446.
[58] DEODHAR A, BLAUVELT A, LEBWOHL M, et al. Long-term safety of Ixekizumab in adults with psoriasis, psoriatic arthritis, or axial spondyloarthritis: a post-hoc analysis of final safety data from 25 randomized clinical trials. Arthritis Res Ther. 2024;26(1):49.
[59] CARNAZZO V, REDI S, BASILE V, et al. Calprotectin: two sides of the same coin. Rheumatology. 2024; 63(1):26-33.
[60] KAZAKOV AS, RASTRYGINA VA, VOLOGZHANNIKOVA AA, et al. Recognition of granulocyte-macrophage colony-stimulating factor by specific S100 proteins. Cell Calcium. 2024;119:102869.
|