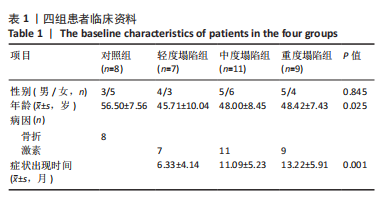
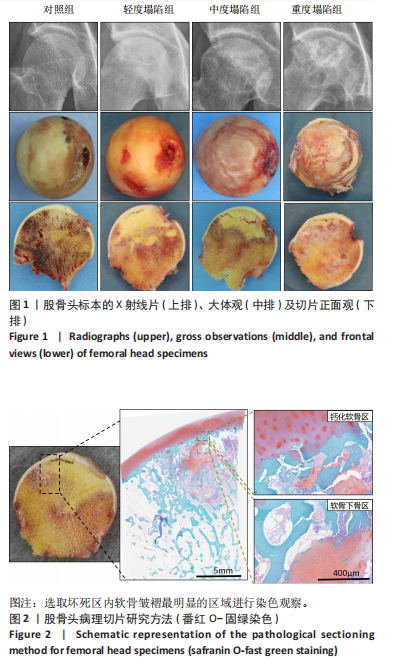
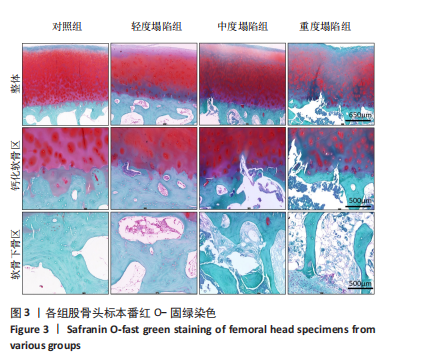
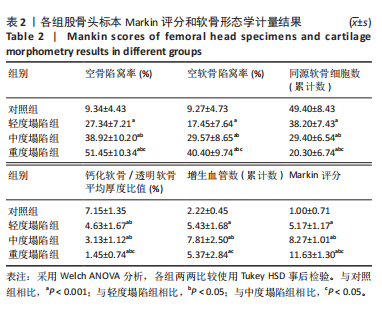
[1] DIMA A, PEDERSEN AB, PEDERSEN L, et al. Association of common comorbidities with osteonecrosis: a nationwide population-based case-control study in Denmark. BMJ Open. 2018;8(2):e020680.
[2] MIMURA N, IWAMOTO T, FURUTA S, et al. Prevalence and risk factors of osteonecrosis of the femoral head in patients with ANCA-associated vasculitis: a multicentre cohort study. RMD Open. 2023;9(1):e002787.
[3] KWON HM, HAN M, LEE TS, et al. Effect of Corticosteroid Use on the Occurrence and Progression of Osteonecrosis of the Femoral Head: A Nationwide Nested Case-Control Study. J Arthroplasty. 2024; 39(10):2496-2505.e1.
[4] SHIMIZU H, SHIMIZU T, TAKAHASHI D, et al. Corticosteroid dose increase is a risk factor for nonalcoholic fatty liver disease and contralateral osteonecrosis of the femoral head: a case report. BMC Musculoskelet Disord. 2019;20(1):88.
[5] LOU P, ZHOU G, WEI B, et al. Sclerotic zone in femoral head necrosis: from pathophysiology to therapeutic implications. EFORT Open Rev. 2023;8(6):451-458.
[6] YIXUAN H, XINWEI Y, FEIFEI G, et al. Effect of Sclerosis Bands in Femoral Head Necrosis on Non-Vascularized Fibular Grafting-A Finite Element Study. Orthop Surg. 2024;16(10):2526-2538.
[7] LIU LH, ZHANG QY, SUN W, et al. Corticosteroid-induced Osteonecrosis of the Femoral Head: Detection, Diagnosis, and Treatment in Earlier Stages. Chin Med J (Engl). 2017;130(21):2601-2607.
[8] NAKAMURA J, FUKUSHIMA W, ANDO W, et al. Time elapsed from definitive diagnosis to surgery for osteonecrosis of the femoral head: a nationwide observational study in Japan. BMJ Open. 2024; 14(3):e082342.
[9] CHENG L, WANG S. Correlation between bone mineral density and sarcopenia in US adults: a population-based study. J Orthop Surg Res. 2023;18(1):588.
[10] LI WL, TAN B, JIA ZX, et al. Exploring the Risk Factors for the Misdiagnosis of Osteonecrosis of Femoral Head: A Case-Control Study. Orthop Surg. 2020;12(6):1792-1798.
[11] CHANG C, GREENSPAN A, GERSHWIN ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. 2020;110:102460.
[12] RAKHSHANKHAH N, ABBASZADEH M, KAZEMI A, et al. Deep learning approach to femoral AVN detection in digital radiography: differentiating patients and pre-collapse stages. BMC Musculoskelet Disord. 2024;25(1):547.
[13] NAM KW, KIM YL, YOO JJ, et al. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90(3): 477-484.
[14] MONT MA, ZYWIEL MG, MARKER DR, et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92(12): 2165-2170.
[15] LIEBERMAN JR, ENGSTROM SM, MENEGHINI RM, et al. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res. 2012;470(2):525-534.
[16] MONT MA, CHERIAN JJ, SIERRA RJ, et al. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A Ten-Year Update. J Bone Joint Surg Am. 2015;97(19):1604-1627.
[17] 魏秋实,庞凤祥,陈哓俊,等.经髋关节外科脱位打压植骨支撑术治疗ARCO Ⅲ期股骨头坏死的临床疗效分析[J].中华损伤与修复杂志(电子版),2020,15(2):90-95.
[18] WEI W, TAN B, YAN Y, et al. Hip Preservation or Total Hip Arthroplasty? A Retrospective Case-Control Study of Factors Influencing Arthroplasty Decision-Making for Patients with Osteonecrosis of the Femoral Head in China. Orthop Surg. 2023;15(3):731-739.
[19] 中国医师协会骨科医师分会骨循环与骨坏死专业委员会,中华医学会骨科分会骨显微修复学组,国际骨循环学会中国区.中国成人股骨头坏死临床诊疗指南(2020)[J].中华骨科杂志,2020, 40(20):12.
[20] 中国微循环学会骨微循环专业委员会,徐鑫,时利军,等.股骨头坏死临床诊疗技术专家共识(2022年)[J].中国修复重建外科杂志, 2022,36(11):1319-1326.
[21] 中华医学会骨科学分会创伤骨科学组,中国医师协会骨科医师分会创伤专家工作委员会.成人股骨颈骨折诊治指南[J].中华创伤骨科杂志,2018,20(11):921-928.
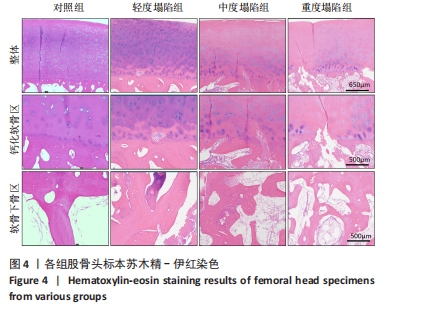
[22] HE Y, GRAM A, SIMONSEN O, et al. AB0104 Develop and Evaluate a New Modified Mankin Score System with Special Attention to Subchondral Bone. Ann Rheum Dis. 2014;73(Suppl 2):838.
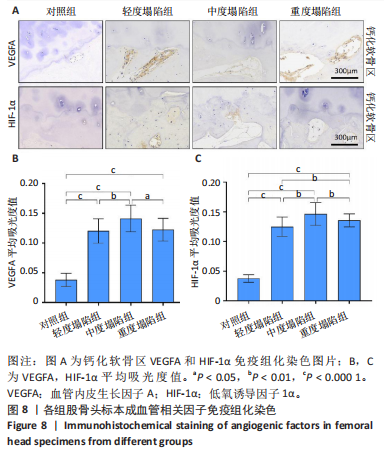
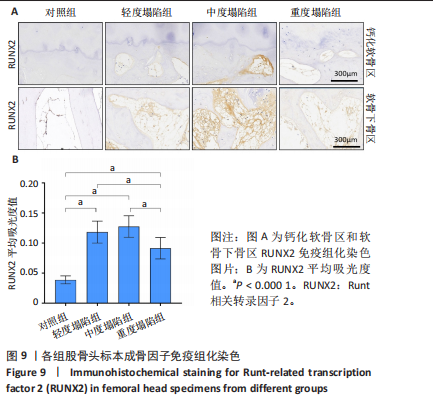
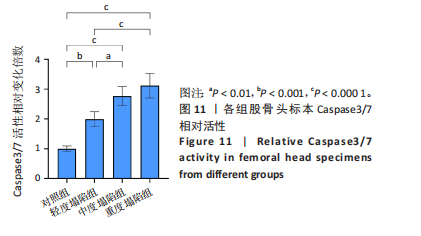
[23] RIZZARDI AE, JOHNSON AT, VOGEL RI, et al. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn Pathol. 2012;7:42.
[24] YOON BH, MONT MA, KOO KH, et al. The 2019 Revised Version of Association Research Circulation Osseous Staging System of Osteonecrosis of the Femoral Head. J Arthroplasty. 2020;35(4):933-940.
[25] ZHANG QY, LI ZR, GAO FQ, et al. Pericollapse Stage of Osteonecrosis of the Femoral Head: A Last Chance for Joint Preservation. Chin Med J (Engl). 2018;131(21):2589-2598.
[26] 魏秋实,何伟,张庆文,等.围塌陷期股骨头坏死不同影像学表现研究[J].中国修复重建外科杂志,2021,35(9):1105-1110.
[27] HATANAKA H, MOTOMURA G, IKEMURA S, et al. Volume of hip synovitis detected on contrast-enhanced magnetic resonance imaging is associated with disease severity after collapse in osteonecrosis of the femoral head. Skeletal Radiol. 2019;48(8):1193-1200.
[28] 魏秋实,杨帆,陈哓俊,等.激素性与酒精性股骨头坏死患者骨标本坏死区域病理与显微结构特点分析[J].中国修复重建外科杂志,2018,32(7):866-872.
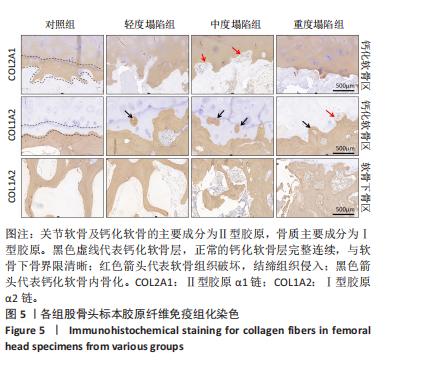
[29] 张德志,胡蕴玉,费正奇,等.激素性骨坏死关节软骨下皮质骨病理改变的实验研究[J].中国矫形外科杂志,2006,14(10):769-771.
[30] MA J, GE J, CHENG L, et al. Subchondral Bone Plate Classification: A New and More Sensitive Approach for Predicting the Prognosis of Osteonecrosis of the Femoral Head. Cartilage. 2023;14(3):269-277.
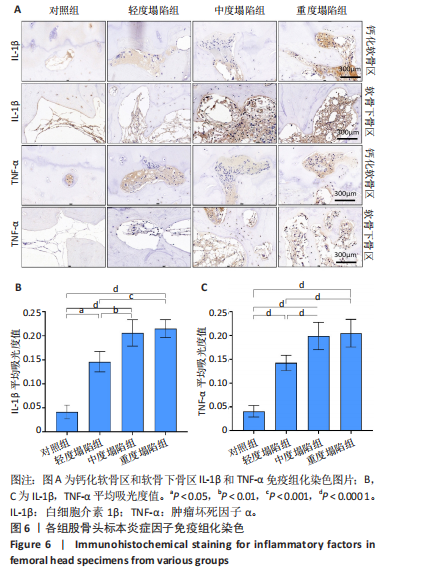
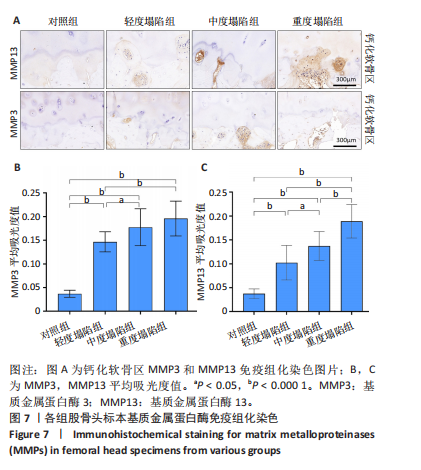
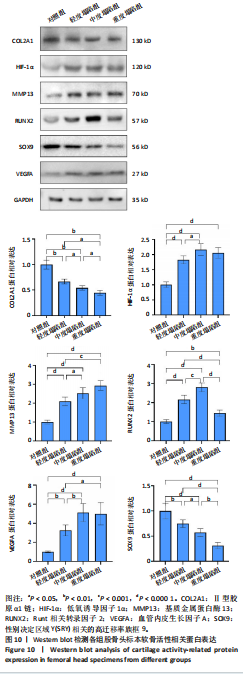
[31] MURPHY G, LEE MH. What are the roles of metalloproteinases in cartilage and bone damage? Ann Rheum Dis. 2005;64 Suppl 4(Suppl 4):iv44-47.
[32] GOLDRING MB, OTERO M, PLUMB DA, et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater. 2011;21:202-220.
[33] WOO S, LEE Y, SUN D. A Pilot Experiment to Measure the Initial Mechanical Stability of the Femoral Head Implant in a Cadaveric Model of Osteonecrosis of Femoral Head Involving up to 50% of the Remaining Femoral Head. Medicina (Kaunas). 2023;59(3):508.
[34] HALL M, VAN DER ESCH M, HINMAN RS, et al. How does hip osteoarthritis differ from knee osteoarthritis? Osteoarthritis Cartilage. 2022;30(1):32-41.
[35] WANG C, MENG H, WANG Y, et al. Analysis of early stage osteonecrosis of the human femoral head and the mechanism of femoral head collapse. Int J Biol Sci. 2018;14(2):156-164.
[36] CHEN Y, MIAO Y, LIU K, et al. Less sclerotic microarchitecture pattern with increased bone resorption in glucocorticoid-associated osteonecrosis of femoral head as compared to alcohol-associated osteonecrosis of femoral head. Front Endocrinol (Lausanne). 2023; 14:1133674.
[37] CHEN Y, YU Y, WEN Y, et al. A high-resolution route map reveals distinct stages of chondrocyte dedifferentiation for cartilage regeneration. Bone Res. 2022;10(1):38.
[38] GOLDRING SR, GOLDRING MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632-644.
[39] FONDI C, FRANCHI A. Definition of bone necrosis by the pathologist. Clin Cases Miner Bone Metab. 2007;4(1):21-26.
[40] EVANS LAE, PITSILLIDES AA. Structural clues to articular calcified cartilage function: A descriptive review of this crucial interface tissue. J Anat. 2022;241(4):875-895.
[41] MAHJOUB M, BERENBAUM F, HOUARD X. Why subchondral bone in osteoarthritis? The importance of the cartilage bone interface in osteoarthritis. Osteoporos Int. 2012;23 Suppl 8:S841-846.
[42] IMHOF H, BREITENSEHER M, KAINBERGER F, et al. Importance of subchondral bone to articular cartilage in health and disease. Top Magn Reson Imaging. 1999;10(3):180-192.
[43] BOYDE A. The Bone Cartilage Interface and Osteoarthritis. Calcif Tissue Int. 2021;109(3):303-328.
[44] PARK S, BELLO A, ARAI Y, et al. Functional Duality of Chondrocyte Hypertrophy and Biomedical Application Trends in Osteoarthritis. Pharmaceutics. 2021;13(8):1139.
[45] DENG B, WANG F, YIN L, et al. Quantitative study on morphology of calcified cartilage zone in OARSI 0~4 cartilage from osteoarthritic knees. Curr Res Transl Med. 2016;64(3):149-154.
[46] WANG X, WU Q, ZHANG R, et al. Stage-specific and location-specific cartilage calcification in osteoarthritis development. Ann Rheum Dis. 2023;82(3):393-402.
[47] 陈雷雷,何伟.股骨头缺血性坏死相关生物力学研究进展[J].中国骨伤,2011,24(2):174-177.
[48] WANG P, WANG C, MENG H, et al. The Role of Structural Deterioration and Biomechanical Changes of the Necrotic Lesion in Collapse Mechanism of Osteonecrosis of the Femoral Head. Orthop Surg. 2022;14(5):831-839.
[49] YU Y, WANG S, ZHOU Z. Cartilage Homeostasis Affects Femoral Head Necrosis Induced by Methylprednisolone in Broilers. Int J Mol Sci. 2020;21(14):4841.
[50] CHEN L, HONG G, FANG B, et al. Predicting the collapse of the femoral head due to osteonecrosis: From basic methods to application prospects. J Orthop Translat. 2017;11:62-72.
[51] LIU Y, MA Y, YANG W, et al. Integrated proteomics and metabolomics analysis of sclerosis-related proteins and femoral head necrosis following internal fixation of femoral neck fractures. Sci Rep. 2024; 14(1):13207.
[52] WANG Y, SUN D, ZHANG J, et al. Multi-sequence MRI-based radiomics: An objective method to diagnose early-stage osteonecrosis of the femoral head. Eur J Radiol. 2024;177:111563.
[53] HAN X, HONG G, GUO Y, et al. Novel MRI technique for the quantification of biochemical deterioration in steroid-induced osteonecrosis of femoral head: a prospective diagnostic trial. J Hip Preserv Surg. 2021;8(1):40-50.
[54] OUYANG W, GUO G, XIA J, et al. Arthroscopic assisted versus open core decompression for osteonecrosis of the femoral head: A systematic review and meta-analysis. PLoS One. 2024;19(11):e0313265.
[55] 林天烨,吴智明,张文胜,等.复方生脉成骨胶囊修复激素性股骨头坏死的作用机制[J].中国组织工程研究,2024,28(2):200-207. |