中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (1): 194-203.doi: 10.12307/2025.570
• 干细胞综述 stem cell review • 上一篇 下一篇
间充质干细胞来源外泌体分离、鉴定技术及应用
刘 宇1,龚森怡1,杨丽华1,李伟风1,胡玉雯1,闫钦彪1,2,郭美锦1
- 华东理工大学,1生物反应器工程国家重点实验室,2商学院,上海市 200237
-
收稿日期:2024-09-25接受日期:2024-11-22出版日期:2026-01-08发布日期:2025-07-02 -
通讯作者:郭美锦,博士,教授,华东理工大学生物反应器工程国家重点实验室,上海市 200237 -
作者简介:刘宇,男,2000年生,江苏省南京市人,汉族,华东理工大学在读硕士,主要从事干细胞外泌体培养及应用相关研究。 -
基金资助:中国科学院战略先导项目(XDA16030702),项目参与者:郭美锦
Isolation, identification, and application of exosomes derived from mesenchymal stem cells
Liu Yu1, Gong Senyi1, Yang Lihua1, Li Weifeng1, Hu Yuwen1, Yan Qinbiao1, 2, Guo Meijin1
- 1State Key Laboratory of Bioreactor Engineering, 2School of Business, East China University of Science and Technology, Shanghai 200237, China
-
Received:2024-09-25Accepted:2024-11-22Online:2026-01-08Published:2025-07-02 -
Contact:Guo Meijin, PhD, Professor, State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, China -
About author:Liu Yu, Master candidate, State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, China -
Supported by:Strategic Priority Research Program of Chinese Academy of Sciences, No. XDA16030702 (to GMJ)
摘要:
文题释义:
间充质干细胞:是一种具有自我更新能力和多向分化潜能的干细胞,存在于脂肪、脐带和骨髓等多种组织和器官中,对机体修复和免疫调节有重要作用,在组织工程和再生医学中有广泛的应用前景。外泌体:是一种直径在30-150 nm的细胞外囊泡,由细胞在正常或异常状态下分泌,能够传递蛋白质、核酸和脂质等生物活性物质至靶细胞以介导细胞间通讯,从而调节细胞与组织的功能。
摘要
背景:间充质干细胞外泌体因低免疫原性和靶向递送效果在细胞通讯和表观遗传调控中发挥重要作用,已在疾病的治疗中临床应用。
目的:综述间充质干细胞外泌体的分离纯化、鉴定方法及应用进展,促进间充质干细胞外泌体大规模制备技术的开发与临床转化。
方法:以“外泌体,间充质干细胞,分离纯化,表征,临床应用”为中文检索词,“exosome,extracellular vesicles,mesenchymal stem cells,isolation,characterization,application”为英文检索词,检索中国知网、PubMed和Web of Science数据库于2024年9月之前发表的文献,排除与主题相关性较差、年代久远及重复的文章,最后纳入109篇文献进行综述。
结果与结论:①综述了近年来外泌体分离纯化方法,根据原理的不同比较了超速离心法、超滤法、尺寸排阻色谱法、聚合物沉淀法、免疫亲和法、微流控法及其他新方法的特点;②外泌体的鉴定方法可以分为物理与生化分析,从外泌体的形状大小及特征蛋白等方面进行表征;③间充质干细胞外泌体因具有免疫调节、跨越生物屏障等能力在医疗美容、损伤修复、癌症治疗等多个领域具有广泛应用;④外泌体由于自身结构复杂、缺少通用分离技术、稳定性较差等缺点而难以在短时间内实现临床转化。
中图分类号:
引用本文
刘 宇, 龚森怡, 杨丽华, 李伟风, 胡玉雯, 闫钦彪, 郭美锦. 间充质干细胞来源外泌体分离、鉴定技术及应用[J]. 中国组织工程研究, 2026, 30(1): 194-203.
Liu Yu, Gong Senyi, Yang Lihua, Li Weifeng, Hu Yuwen, Yan Qinbiao, Guo Meijin. Isolation, identification, and application of exosomes derived from mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 194-203.
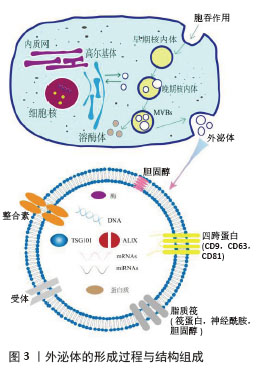
具有球形外观、直径在30-150 nm,且包含多种小分子生物活性物质[7],见图3。因其特殊的物理化学结构,外泌体可以携载核酸及蛋白质等活性成分从供体细胞转移到受体细胞,介导细胞间的信息交换[8],这与外泌体形成密切相关。外泌体的形成早期是通过质膜向内出芽得到早期内小体,这是一种膜结构的囊腔;游离的泛素化蛋白质和其他核酸分子通过特定膜融合途径进入早期小体的囊腔中形成晚期小体,此时为了进一步释放外泌体,晚期小体向细胞膜移动并对囊体膜进行修饰以更好地与细胞膜融合释放外泌体。在此过程中,不同细胞环境中存在的特有标志蛋白也被包裹在外泌体中,作为标志物应用于特定靶点的研究[9]。
2.2 外泌体分离纯化方法发展 为了促进外泌体在临床研究中的应用,规模化制备外泌体并提高其纯度是首要前提。以下对外泌体分离方法的原理、流程与特点进行介绍,见图4。
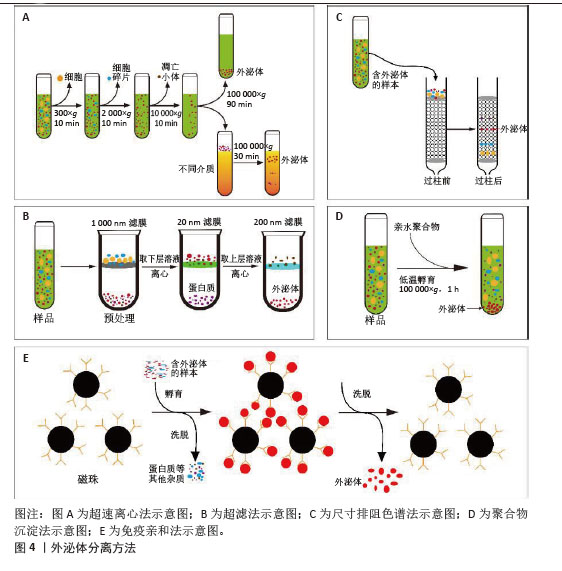
2.2.1 超速离心法 超速离心法因能够稳定地从几乎所有的样品中分离出纯度较高的外泌体而被当作外泌体分离的“金标准”[10],因其适用范围非常广泛、设备与操作的要求较为单一和外泌体纯度高,已成为实验人员首选的外泌体分离方案。超速离心法一般可以分为2类:差速超速离心法和密度梯度离心法。差速超速离心通过不同离心力和持续循环数,将外泌体与样品中其他密度和大小差异的成分分开[11];而密度梯度离心则是在其基础上使用2种或2种以上不同密度的分离介质(如蔗糖和碘二醇)进行逐层分离[12]。然而研究发现,长时间离心处理会使外泌体受到明显的机械损伤,虽然超速离心可以得到高浓度的外泌体,但它耗时较长,难以在短时间内处理多个生物样品[13],且对外泌体的损伤也不容忽视。此外,超速离心法的富集功能可能会使外泌体在制备过程中受到可溶性蛋白污染[14]。
2.2.2 超滤法 超滤的基本原理与传统的膜过滤相同,其中悬浮颗粒或聚合物的分离主要取决于它们的大小或分子质量。因此,根据外泌体的大小,可以使用具有确定分子质量或粒径大小限制的膜过滤器分离外泌体[15]。但是小规格的滤器很容易发生囊泡堵塞,降低膜的使用寿命并导致分离率降低[16]。除了用蛋白酶预处理以降低样品黏度外[17],切向流过滤技术是解决这一问题的理想方法[18]。在切向流过滤过程中,进料流与膜平行流动[19],通过操纵流体动力,施加在流体上的压力使只有一部分流体穿过膜,可以有效地减少潜在的堵塞问题,剩余的滞留物可以通过再循环回到进料储罐中进行重复过滤,从而实现自动化过程和高产量[20]。虽然基于这些优点,切向流技术已经广泛用来过滤分离外泌体,但在重复循环过程中也使得更多的杂质有机会通过滤膜,降低最终产物的纯度。因此,进一步优化切向流过滤中多次循环导致的纯度降低问题有望实现该方法的规模化应用。
2.2.3 尺寸排阻色谱法 与超滤法原理相似,尺寸排阻色谱(Size exclusion chromatography,SEC)是一种基于重力差异的分离技术。将样品加入到含有多孔珠的分离柱中,较大的颗粒无法进入凝胶孔,洗脱速度更快[21];相反,小颗粒可以进入孔隙,洗脱速度较慢,最终达到分离的目的。与超速离心法和超滤法不同,尺寸排阻色谱是通过被动重力流进行的,不影响囊泡结构和完整性[22],还可以通过选择具有生理渗透压和黏度的洗脱缓冲液进一步稳定外泌体的自然状态[23]。然而,目前常见的尺寸排阻色谱设备均存在较长的处理时间和低回收率等问题,明显限制了尺寸排阻色谱技术的大规模使用。
2.2.4 聚合物沉淀法 聚合物诱导沉淀法也是常用的外泌体分离策略之一。高度亲水性聚合物与外泌体周围的水分子相互作用,形成疏水微环境,导致外泌体沉淀[24]。聚乙二醇是一种良好的无毒聚合物,具有重塑周围材料水溶性的能力,已被广泛用于外泌体分离[25]。聚合物沉淀法实验步骤简单、成本低,适合处理大体积样品同时提高收率,曾被许多实验人员优先采纳[26]。但随着外泌体应用研究的进一步深入,对外泌体纯度的要求越来越高,而沉淀法富集的不仅包含外泌体,还有其他高分子杂质,缺乏样品选择性。当然,在实验室小规模的外泌体分离中,沉淀法的简便稳定与高产量依旧在广泛应用。
2.2.5 免疫亲和法 免疫亲和法依赖于外泌体表面表达的特异性蛋白抗体来进行捕获而分离。针对外泌体的特异性蛋白抗体可以附着在平板、磁珠、树脂和微流体装置等不同基质上,进而从样品中捕获含有特异性蛋白的外泌体[27]。与其他技术相比,免疫法虽然特异性高,捕获的外泌体纯度更高,但产量相对更低[28]。由于生物液体的复杂性,免疫亲和捕获技术常用于外泌体超速离心或超滤富集后,非常适合临床诊断等少量样品的应用场景,既获得高纯度的同时,还可以保留外泌体的活性[29]。在实际应用研究中,发现高特异性且低成本的标志蛋白抗体是免疫法分离外泌体的主要困难。此外,外泌体含有多个标志蛋白,且不一定同时同体表达,通过抗原抗体结合捕获提取液中的所有外泌体效率不高。未来有望通过寻找外泌体表面高表达蛋白抗体或优化捕获效率提高抗体使用频率的方法加大免疫法在外泌体分离的应用。
2.2.6 微流控技术 微流控技术是将样品处理、分析、检测等过程集成到一个芯片上,具有小型化、集成化、高通量、低耗时等特点[30]。目前,微流控芯片正逐渐成为简便、高效地分离外泌体的有力工具,最大特点是将外泌体分离和表征转变为一步过程[31]。其中,外泌体分离部分主要是通过免疫亲合法结合一种根据物理性质分离方法而进行分离[32]。此外,微流控装置的另一个作用是外泌体的分析鉴定,它通过一种高灵敏度、高通量的微流体装置对单个外泌体精度分析和表征,即在有效地分离高纯度的外泌体并及时地表征外泌体数据,从而利于提高不同亚群外泌体的鉴定[33]。虽然该方法的成本较高和处理量较小,但目前小剂量的处理在临床诊断领域非常适用。随着微流控技术的发展,大规模制备外泌体的微流控系统也会成为可能。
2.2.7 组合分离纯化法 目前常用的外泌体分离方法在单一使用时难以同时达到大规模、高纯度的要求,因此不同方法之间的联用可以有效弥补各自的不足。如将超速离心、超滤、尺寸排阻色谱、聚合物沉淀和免疫亲和法中的2种或多种方法结合[34-36],建立更高效的外泌体生产工艺。例如:聚合物沉淀与切向流过滤及尺寸排阻色谱法的结合可以降低提取过程中脂蛋白的含量而提高最终产物的纯度[37];免疫亲和法与尺寸排阻色谱法联合分离,更精确地从血清中分离出外泌体[38];超滤结合尺寸排阻色谱法更高效地分离出柑橘柠檬汁中的外泌体[39]。虽然不同方法的组合增加了实验成本、分离时间以及操作步骤,但对于制备特殊纯度需求的外泌体,工艺联用的组合有望稳定简便地分离大量高纯度外泌体。
2.2.8 其他分离方法 除了上述常用的外泌体分离方法外,近来流体力学和阴阳离子结合也被应用于外泌体的纯化中,这些新方法的尝试提高了外泌体分离的多样性,为未来开发出高效稳定地外泌体分离工艺提供了新方法。
非对称流场流动分馏法(AsFIFFF4, AF4)通过2块平板形成通道,上壁为不透水的聚碳酸酯玻璃板,下通道板为透气的由多孔不锈钢熔块材料制成,中间设有聚酯梯形分离器和超滤膜,底流道板与超滤膜可形成团聚壁[40]。该技术是一种温和的分离策略,没有强大的剪切力,因此有利于保持外泌体的天然结构和生物完整性,并允许分离外泌体亚型。然而,该技术需要特定的设备和专业人员来调节和优化横流速度、通道高度和膜类型等各种参数。 此外,AF4不适合大体积样品,需要超速离心、超滤等方法进行预浓缩[41]。
离子交换法依赖于外泌体膜的Zeta负电位与带正电荷的阴离子交换基质的相互作用进行结合,再通过提高盐浓度等方法增加缓冲离子强度进行分离[42]。该方法具有较高的可操作性和可扩展性,且产物纯度可以联合超滤等预处理方法进行提升。然而,因许多生物样本含有复杂的成分和带电物质不明确,离子交换法目前主要用于细胞培养基的样本处理。
各种外泌体分离方法的优势与局限性列于表1。
2.3 外泌体鉴定方法 深入研究外泌体的生物特性与作用机制,对其形态特征与组成成分的分析至关重要。目前对于外泌体的分析方法分为2类:物理分析与生化分析。物理分析通过观察外泌体的形态分布、颗粒大小与浓度,使用的设备和方法包括透射电子显微镜(TEM)、纳米颗粒跟踪分析(NTA)和动态光散射(DLS)等。生化分析通过使用纳米流式细胞术、蛋白免疫印迹法(Western blotting)、qPCR及蛋白质组学等分析外泌体的组成。
2.3.1 物理分析 外泌体的形貌是其基本形态表征之一,透射电子显微镜是对外泌体整体形态进行清晰和直观观察最常用的方法[51]。透射电子显微镜是利用高能电子束充当照明光源而进行放大成像的大型显微分析设备,可以观察纳米级细微结构,分辨率可达到0.2 nm。外泌体的形状是杯状或茶托形,透射电子显微镜可以直观地看到粒子的形态,鉴定外泌体的存在和完整性[52]。但电镜只能展示局部景象,无法区分与外泌体形态相近的纳米粒子,因此不能反映样品中的外泌体数量,需要与其他测试仪器相互验证进行判断。
纳米颗粒跟踪分析是检测外泌体粒径与颗粒浓度最高效的方法[53],其原理是利用光散射和布朗运动的特性获得悬浮液中的样本粒度分布。将一束能量集中的激光穿过玻璃棱镜,激光光束从较小角度入射进入样品溶液,照亮溶液中的颗粒,再通过收集散射的激光束,获得样品粒度分布的信息[54]。它能对悬浮液中粒径分布范围较宽的颗粒进行全方位表征,具有分辨率高、检测速度快、准确度高等优点。然而,纳米颗粒跟踪分析的结果仅仅是样品中的粒子信息,无法判断粒子的具体种类。因此,在实际应用过程中由于分离的外泌体样品纯度不一,只通过纳米颗粒跟踪分析很难分析外泌体的具体单位体积浓度。
动态光散射法是用于确定溶液样品中悬浮体或聚合物中颗粒尺寸和半径分布最常用的分析方法之一[55]。单色光束照射到含有以布朗运动形式移动的球形粒子的溶液中,当光击中移动的粒子时会引起多普勒频移,可以计算出颗粒的尺寸分布并详细描述颗粒在被测介质中的运动[56]。与纳米颗粒跟踪分析相比,动态光散射只能提供大概的粒径范围,不能检测溶液中的具体颗粒数,因此在外泌体表征过程中不被优先考虑。但动态光散射具有检测精度高、样品制备容易、操作简单和检测范围广等优点。研究发现先用动态光散射比较样品间的差异,再使用纳米颗粒跟踪分析得出详细粒子分布与浓度,可以有效节约时间、降低成本。
2.3.2 生化分析 在进行外泌体的表征时,仅仅通过物理分析方法难以准确判断外泌体的浓度,因此对外泌体的蛋白组成分析是很有必要的。根据《Minimal information for studies of extracellular vesicles 2018》(MISEV2018)指南推荐,Particle(粒子数)/蛋白可以作为外泌体纯度的指标,且这一标准在实际研究当中得到广泛运用。
蛋白免疫印迹是鉴定外泌体特征蛋白最常用和成熟的技术,其将蛋白样本通过聚丙烯酰胺电泳按分子质量大小分离,再转移到硝化纤维素或聚偏氟乙烯(PVDF)膜上,然后通过相应抗体对靶蛋白进行特异性检测[57]。研究发现,人源外泌体的标志性蛋白一般选择CD9、CD63以及CD81等标记物,它们是外泌体膜上常见的四跨蛋白,在膜融合、信号传导和蛋白质运输中发挥作用[58];此外,Alix、Tsg101蛋白也参与外泌体有关生物发生[59]。但是目前已经鉴定到的这些标志物蛋白并不是外泌体所特有的,诸如CD63、Alix等在细胞中广泛存在,在一些细胞碎片、溶酶体、胞内体中也大量存在。因此,蛋白免疫印迹仅能证实外泌体成分的存在,无法确认外泌体的数量和完整性。
流式细胞术常常用于表征细胞等大颗粒,通过识别荧光标记可以准确计算出样品中目的颗粒的含量。然而,传统的流式细胞术在检测直径< 500 nm的小颗粒时灵敏度和分辨率有限,造成外泌体颗粒识别不全,导致误差非常大[60]。为了使传统的流式细胞术适应外泌体分析,使用微米大小的乳胶珠来结合多个外泌体,并用荧光抗体对结合的外泌体进行染色,对其蛋白质标记物进行表征可以解决这一问题[61]。然而这种方法缺乏对单个外泌体分析能力,难以做到对外泌体亚群的准确区分。因此在此基础上开发了纳米流式仪,其可以区分直径小至100 nm的颗粒,从而提高了外泌体分析的准确性[62]。不过纳米流式仪识别能力非常依赖外泌体的相应蛋白表达,由于大多数外泌体的特征蛋白不会在所有的外泌体上同时表达,且表达量太少的蛋白会导致外泌体难以被识别,即使先进的纳米流式也很难做到真正意义上的准确区分不同亚型的外泌体。
除了鉴定外泌体中已知的特征蛋白外,外泌体中不同丰度的未知蛋白也可以通过蛋白质组学进行检测。纳升液质联用仪融合了双压离子阱的速度和灵敏度以及Orbitrap技术的高分辨率和出色质量精度,能够对复杂生物样品进行高分辨率的深度分析,可用于蛋白质组学高通量蛋白定性定量检测解析[63]。基于质谱的蛋白质组学技术有助于揭示外泌体中不同蛋白质的目的和活性,以及它们与亲本细胞中的蛋白质的相似和不同之处。
外泌体除了丰富的蛋白质外,也含有多种RNA和miRNA,通过表面蛋白与靶点的结合,释放体内遗传信息到特定受体细胞,进行表达蛋白的转录和翻译。琼脂糖凝胶电泳是一种电泳形式,用于根据核酸片段的大小进行分离。在研究外泌体对表达基因的影响时,还需要用到qPCR[64]、RT-qPCR等技术对核酸进行分离鉴定[65]。
总的来说,现今的外泌体鉴定方法缺乏统一和标准化检测要求,在越来越多的研究当中发现,无论是外泌体表观形貌的鉴定还是跨膜蛋白的检测,都不能通过单一方法进行判断是否为外泌体,尤其是外泌体的浓度和纯度。但是,总结目前的研究可以发现颗粒分布大小、表观形貌和特征蛋白是现在常用的外泌体鉴定的基本组合,能有效确定外泌体的存在并进行基本定量分析。表2比较了外泌体鉴定方法。
2.4 间充质干细胞外泌体的临床应用 即使外泌体的分离与分析方法尚不完善,但相关应用的研究已逐步开展,从中国临床试验注册中心查询到16个与间充质干细胞外泌体(MSCs-Exo)相关的临床试验,部分代表性临床试验见表3。与直接应用间充质干细胞的治疗方案相比,其分泌的外泌体在再生医学领域有着独特的优势。首先,间充质干细胞外泌体具有显著的免疫调节能力[71],可以影响免疫细胞的活性、抑制炎症,避免了间充质干细胞治疗过程中的免疫原性相关的问题[72]。受限于细胞培养本身的复杂性,外泌体更容易完成标准化与规模化生产,从而促进临床转化的进程[73]。此外,与干细胞在治疗过程中存在的致瘤性与细胞分化的风险相比,外泌体发生免疫排斥或引发肿瘤的可能性更小[74],而且外泌体的靶向效果更强,可以装载药物并跨越生物屏障[75]。这些优势为间充质干细胞外泌体治疗方案的开发奠定了基础,目前已在部分疾病领域得到应用,见图5。
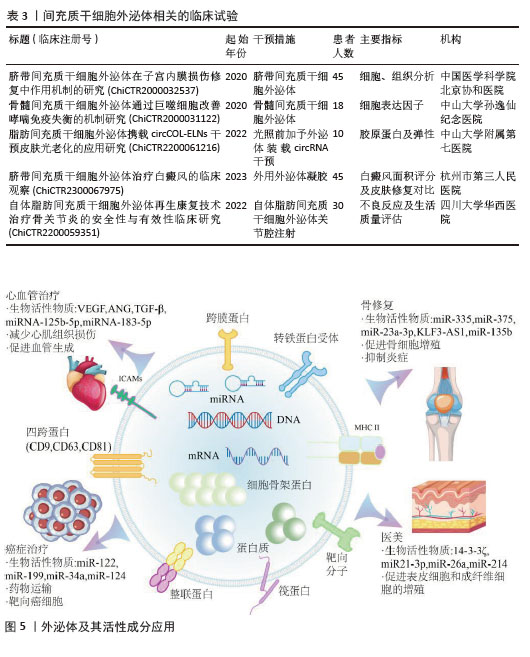
2.4.1 医疗美容 衰老是生命不可避免的自然过程,它导致组织和器官的结构功能退化。皮肤作为人体最大的器官,不可避免地会面临与衰老相关的问题[76]。在体内移植干细胞可显著延缓衰老,其旁分泌的外泌体是实现该功能的重要因素[77]。研究证明间充质干细胞来源的外泌体可以通过调控14-3-3ζ、基质金属蛋白酶和NOX1等蛋白及ERK-1/2[78]、Wnt/β-catenin等通路促进人皮肤角质形成细胞(HaCat)的增殖和迁移[79],恢复细胞外基质成分[80],降低活性氧水平[81],最终逆转皮肤衰老。此外间充质干细胞外泌体携带的多种miRNA与生长因子可以有效降低皮肤中黑色素水平并促进伤口愈合[82],为其开发医疗美容领域的市场提供了强力的支持。
2.4.2 骨损伤修复 外泌体中包含着种类丰富的遗传物质,对于成骨所需的生长因子的表达影响深远,也是外泌体在骨组织修复过程中发挥作用的重要前提[83]。间充质干细胞外泌体通过其携带的miRNA及抗炎因子等活性物质促进软骨细胞和骨髓间充质干细胞的增殖、迁移和分化[84],刺激巨噬细胞向抗炎M2表型极化,发挥炎症抑制作用,从而缓解关节炎[85]。诸多研究表明间充质干细胞外泌体促进新骨生长与血管生成,对成骨有关的细胞发挥积极作用,从而促进骨修复进程[86]。
2.4.3 神经治疗 神经性疾病由于药物作用有限而一直难以治愈,间充质干细胞来源的外泌体含有多种抗凋亡、抗炎和抗氧化成分,在治疗阿尔茨海默症等各种神经疾病方面具有很大的前景[87]。最新的研究表明间充质干细胞外泌体拥有恢复神经元记忆和突触可塑性[88]、减少神经元死亡[89]、促进神经信号传导等功能[90],为神经疾病的治疗提供新的思路。
2.4.4 肠病治疗 炎症性肠病是一种慢性、非特异性的胃肠道炎症性疾病[91],具体发病机制尚不清楚,可能存在遗传、免疫和微生物因素的综合作用[92]。治疗炎症性肠病的临床策略多种多样,如使用免疫调节剂以及微生物移植等方法。由于表观遗传改变与炎症性肠病的发生发展密切相关,外泌体也被广泛用于炎症性肠病的治疗[93]。间充质干细胞外泌体可通过增加总IkBa蛋白和mRNA水平[94]、促进中性粒细胞向N2表型极化[95]、提高细胞存活率及消除细胞焦亡等多种途径调节表观遗传修饰改善炎症,从而缓解炎症性肠病的发展[96]。
2.4.5 心脏病防治 心脏病是导致机体死亡的主要因素,目前还没有药物治疗可以有效地阻止心肌缺血引起的心肌细胞死亡[97]。而间充质干细胞外泌体可以将其携带的生长因子、细胞因子和miRNA等生物活性分子传递给受损心脏组织中的受体细胞,发挥心脏保护特性[98],减少了缺氧诱导的心肌组织损伤[99],预防心肌梗死[100],促进受损的心脏血管生成,为心脏再生、无创诊断、靶向给药、心脏保护和个性化医疗开辟新的途径。
2.4.6 癌症治疗 癌症作为现今世界上发病率与致死率极高的疾病之一,研究人员一直在探索治疗癌症的有效方案。外泌体由于其靶向肿瘤的特异性高、免疫原性低、跨越生物屏障能力强在癌症治疗领域展现出强大的潜力[101]。间充质干细胞外泌体通过高表达miR-122[102]、miR-199[103]、miR-124等关键基因降低肿瘤细胞的抗药性[104],有效加强药物的杀伤能力,同时减少对正常细胞的影响,从而全面提高治疗效果。
2.4.7 药物载体 除了外泌体本身携带的活性物质之外,由于其尺寸极小、生物相容性较好、具有天然靶向性和易于修饰等优点,被认为是一种很有应用前景的药物载体[105]。外泌体通过电穿孔、超声、挤压、冻融等物理方法及表面功能化修饰、基因工程等生化方法装载药物、核酸和蛋白质等有效物质[106],提高治疗方案的效率。基于间充质干细胞外泌体的药物载体在炎症[107]、癌症和免疫缺陷型疾病中得到应用[108-109]。
综上,间充质干细胞来源的外泌体在抗衰、炎症治疗、损伤修复及癌症领域具有潜在的应用前景,这不仅促进研究者们对于间充质干细胞外泌体功能的进一步探索,也有利于推动外泌体分离鉴定方案的开发。
| [1] HOANG DM, PHAM PT, BACH TQ, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1):272. [2] KOU M, HUANG L, YANG JJ, et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 2022;13(7):16. [3] LOTFY A, ABOQUELLA NM, WANG HJ. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14(1):18. [4] LAI JJ, CHAU ZL, CHEN SY, et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv Sci (Weinh). 2022;9(15):e2103222. [5] AGRAWAL AK, AQIL F, JEYABALAN J, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13(5):1627-1636. [6] ARYA SB, COLLIE SP, PARENT CA. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2024;34(2): 90-108. [7] KRYLOVA SV, FENG DR. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci. 2023;24(2):1337. [8] LI YB, HUANG P, NASSER MI, et al. Role of exosomes in bone and joint disease metabolism, diagnosis, and therapy. Eur J Pharm Sci. 2022;176:106262. [9] LIANG YJ, IQBAL Z, LU JP, et al. Cell-derived nanovesicle-mediated drug delivery to the brain: Principles and strategies for vesicle engineering. Mol Ther. 2023;31(5):1207-1224. [10] XU K, JIN YL, LI YM, et al. Recent Progress of Exosome Isolation and Peptide Recognition-Guided Strategies for Exosome Research. Front Chem. 2022:10:844124. [11] HUANG K, GARIMELLA S, CLAY-GILMOUR A, et al. Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. J Pers Med. 2022;12(3):340. [12] WANG WJ, SUN H, DUAN HJ, et al. Isolation and usage of exosomes in central nervous system diseases. CNS Neurosci Ther. 2024; 30(3):e14677. [13] ZABOROWSKI MP, BALAJ L, BREAKEFIELD XO, et al. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience. 2015;65(8):783-797. [14] MARTINS TS, VAZ M, HENRIQUES AG. A review on comparative studies addressing exosome isolation methods from body fluids. Anal Bioanal Chem. 2023;415(7):1239-1263. [15] ANSARI FJ, TAFTI H A, AMANZADEH A, et al. Comparison of the efficiency of ultrafiltration, precipitation, and ultracentrifugation methods for exosome isolation. Biochem Biophys Rep. 2024:38:101668. [16] PETERSON MF, OTOC N, SETHI JK, et al. Integrated systems for exosome investigation. Methods. 2015;87:31-45. [17] GHEINANI AH, VöGELI M, BAUMGARTNER U, et al. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci Rep. 2018;8(1):3945. [18] HUA X, ZHU Q, LIU Y, et al. A double tangential flow filtration-based microfluidic device for highly efficient separation and enrichment of exosomes. Anal Chim Acta. 2023:1258:341160. [19] KO M, KIM HJ, PARK J, et al. Isolation of Bovine Milk Exosome Using Electrophoretic Oscillation Assisted Tangential Flow Filtration with Antifouling of Micro-ultrafiltration Membrane Filters. ACS Appl Mater Interfaces. 2023;15(21):26069-26080. [20] VISAN KS, LOBB RJ, HAM S, et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J Extracell Vesicles. 2022;11(9):e12266. [21] XU WM, LI A, CHEN JJ, et al. Research Development on Exosome Separation Technology. J Membr Biol. 2023;256(1):25-34. [22] ALTINTAS O, SAYLAN Y. Exploring the Versatility of Exosomes: A Review on Isolation, Characterization, Detection Methods, and Diverse Applications. Anal Chem. 2023;95(44): 16029-16048. [23] CHEN JC, LI PL, ZHANG TY, et al. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol. 2022;9:811971. [24] JANG J, JEONG H, JANG E, et al. Isolation of high-purity and high-stability exosomes from ginseng. Front Plant Sci. 2023:13:1064412. [25] SHIEH TM, TSENG YH, HSIA SM, et al. Optimization Protocol of the PEG-Based Method for OSCC-Derived Exosome Isolation and Downstream Applications. Separations. 2022;9(12):11. [26] ZHANG MD, JIN K, GAO L, et al. Methods and Technologies for Exosome Isolation and Characterization. Small Methods. 2018; 2(9):10. [27] TANG Q, XIAO XY, LI RH, et al. Recent Advances in Detection for Breast-Cancer-Derived Exosomes. Molecules. 2022;27(19):6673. [28] PANWAR D, SHRIVASTAVA D, BHAWAL S, et al. Detection of exosomes in various biological fluids utilizing specific epitopes and directed multiple antigenic peptide antibodies. Rev Anal Chem. 2023;42(1):11. [29] GUO XN, HU F, ZHAO SH, et al. Immunomagnetic Separation Method Integrated with the Strep-Tag II System for Rapid Enrichment and Mild Release of Exosomes. Anal Chem. 2023;95(7): 3569-3576. [30] SINGH PK, PATEL A, KAFFENES A, et al. Microfluidic Approaches and Methods Enabling Extracellular Vesicle Isolation for Cancer Diagnostics. Micromachines (Basel). 2022;13(1):139. [31] SHEN JN, MA ZT, XU JQ, et al. Exosome Isolation and Detection: From Microfluidic Chips to Nanoplasmonic Biosensor. ACS Appl Mater Interfaces. 2024 Apr 27. doi: 10.1021/acsami.3c19396. [32] BAI JJ, WEI X, ZHANG X, et al. Microfluidic strategies for the isolation and profiling of exosomes. Trac-Trends Anal Chem. 2023; 158:12. [33] WU YS, WANG YQ, LU YJ, et al. Microfluidic Technology for the Isolation and Analysis of Exosomes. Micromachines (Basel). 2022; 13(10):1571. [34] DING T, CHENG T, ZHU XR, et al. Exosomes mediate the antibody-resistant intercellular transmission of porcine epidemic diarrhea virus. Vet Microbiol. 2023:284:109834. [35] MAHGOUB EO, ABDELLA GM. Improved exosome isolation methods from non-small lung cancer cells (NC1975) and their characterization using morphological and surface protein biomarker methods. J Cancer Res Clin Oncol. 2023;149(10):7505-7514. [36] CHEN ZH, WANG D, GU SS, et al. Size exclusion chromatography and asymmetrical flow field-flow fractionation for structural characterization of polysaccharides: A comparative review. Int J Biol Macromol. 2024;277(Pt 2):134236. [37] IANNOTTA D, A A, LAI AD, et al. Chemically-Induced Lipoprotein Breakdown for Improved Extracellular Vesicle Purification. Small. 2024; 20(18):e2307240. [38] KOKSAL AR, EKMEN N, AYDIN Y, et al. A Single-Step Immunocapture Assay to Quantify HCC Exosomes Using the Highly Sensitive Fluorescence Nanoparticle-Tracking Analysis. J Hepatocell Carcinoma. 2023:10:1935-1954. [39] STEC A, CHODKOWSKA M, KASPRZYK-POCHOPIEN J, et al. Isolation of Citrus lemon extracellular vesicles: Development and process control using capillary electrophoresis. Food Chem. 2023:424:136333. [40] CHEN X, ZHANG WH, DOU YW, et al. Applications of asymmetrical flow field-flow fractionation for separation and characterization of polysaccharides: A review. J Chromatogr A. 2021;1635:461726. [41] JIA YW, YU L, MA TL, et al. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics. 2022;12(15): 6548-6575. [42] ZHANG WY, MENG TJ, HU J, et al. A Liquid Band-Aid with Mesenchymal Stem Cell-Derived Exosomes for Wound Healing in Mice. Curr Pharm Biotechnol. 2024 Sep 30. doi: 10.2174/0113892010331302240913114112. [43] MOGHASSEMI S, DADASHZADEH A, SOUSA MJ, et al. Extracellular vesicles in nanomedicine and regenerative medicine: A review over the last decade. Bioact Mater. 2024;36:126-156. [44] PARK SH, LEE EK, YIM J, et al. Exosomes: Nomenclature, Isolation, and Biological Roles in Liver Diseases. Biomol Ther (Seoul). 2023;31(3):253-263. [45] ZHU FK, WANG TY, WANG GJ, et al. The Exosome-Mediated Bone Regeneration: An Advanced Horizon Toward the Isolation, Engineering, Carrying Modalities, and Mechanisms. Adv Healthc Mater. 2024;13(19): e2400293. [46] SHIREJINI SZ, INCI F. The Yin and Yang of exosome isolation methods: conventional practice, microfluidics, and commercial kits. Biotechnol Adv. 2022:54:107814. [47] KIMIZ-GEBOLOGLU I, ONCEL SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022:347:533-543. [48] KHANABDALI R, MANDREKAR M, GRYGIEL R, et al. High-throughput surface epitope immunoaffinity isolation of extracellular vesicles and downstream analysis. Biol Methods Protoc. 2024;9(1):bpae032. [49] OMRANI M, BEYRAMPOUR-BASMENJ H, JAHANBAN-ESFAHLAN R, et al. Global trend in exosome isolation and application: an update concept in management of diseases. Mol Cell Biochem. 2024;479(3):679-691. [50] BOK EY, SEO SY, LEE HG, et al. Exosomes isolation from bovine serum: qualitative and quantitative comparison between ultracentrifugation, combination ultracentrifugation and size exclusion chromatography, and exoEasy methods. J Anim Sci Technol. 2024;66(5):1021-1033. [51] ISHII N, NOGUCHI K, IKEMOTO MJ, et al. Optimizing Exosome Preparation Based on Size and Morphology: Insights From Electron Microscopy. Microsc Microanal. 2023;29(6): 2068-2079. [52] LIU Z, XUE HW, CHEN Q, et al. A method for extraction of exosomes from breast tumour cells and characterisation by transmission electron microscopy. J Microsc. 2023;292(3):117-122. [53] LOGOZZI M, OREFICE NS, DI RAIMO R, et al. The Importance of Detecting, Quantifying, and Characterizing Exosomes as a New Diagnostic/Prognostic Approach for Tumor Patients. Cancers (Basel). 2023; 15(11):2878. [54] HOFMANN L, MEDYANY V, EZIC J, et al. Cargo and Functional Profile of Saliva-Derived Exosomes Reveal Biomarkers Specific for Head and Neck Cancer. Front Med (Lausanne). 2022:9:904295. [55] XU S T, ZHANG Y X, LIU S L, et al. Exosomes derived from cardiac fibroblasts with angiotensin II stimulation provoke hypertrophy and autophagy inhibition in cardiomyocytes. Biochem Biophys Res Commun. 2023:682: 199-206. [56] HASSAN PA, RANA S, VERMA G. Making Sense of Brownian Motion: Colloid Characterization by Dynamic Light Scattering. Langmuir. 2015; 31(1):3-12. [57] ZHOU XH, XU H, XU C, et al. Hepatocellular carcinoma-derived exosomal miRNA-761 regulates the tumor microenvironment by targeting the SOCS2/JAK2/STAT3 pathway. World J Emerg Med. 2022;13(5):379-385. [58] NAKAMACHI Y, UTO K, HAYASHI S, et al. Exosomes derived from synovial fibroblasts from patients with rheumatoid arthritis promote macrophage migration that can be suppressed by miR-124-3p. Heliyon. 2023; 9(4):e14986. [59] LEE KM, SEO EC, LEE JH, et al. The Multifunctional Protein Syntenin-1: Regulator of Exosome Biogenesis, Cellular Function, and Tumor Progression. Int J Mol Sci. 2023; 24(11):9418. [60] HUICA R, HUICA S, MOLDOVEANU E. Flow cytometric assessment of circulating microparticles - towards a more objective analysis. Rom Biotech Lett. 2011;16(3):6271-6277. [61] ARRAUD N, GOUNOU C, TURPIN D, et al. Fluorescence Triggering: A General Strategy for Enumerating and Phenotyping Extracellular Vesicles by Flow Cytometry. Cytometry A. 2016;89A(2):184-195. [62] LIU HS, TIAN Y, XUE CF, et al. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J Extracell Vesicles. 2022;11(4):e12206. [63] HAN KY, CHANG JH, AZAR DT. Proteomics-Based Characterization of the Effects of MMP14 on the Protein Content of Exosomes from Corneal Fibroblasts. Protein Pept Lett. 2020;27(10):979-988. [64] SHEN ZR, SHAO JJ, SUN JQ, et al. Exosomes released by melanocytes modulate fibroblasts to promote keloid formation: a pilot study. J Zhejiang Univ Sci B. 2022;23(8):699-704. [65] YIN SH, JIA FM, RAN LQ, et al. Exosomes derived from idiopathic gingival fibroma fibroblasts regulate gingival fibroblast proliferation and apoptosis. Oral Dis. 2021; 27(7):1789-1795. [66] WEI HX, CHEN JY, WANG SL, et al. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int J Nanomed. 2019;14:8603-8610. [67] WU SJ, ZHAO YL, ZHANG ZT, et al. The Advances and Applications of Characterization Technique for Exosomes: From Dynamic Light Scattering to Super-Resolution Imaging Technology. Photonics. 2024;11(2):21. [68] AHN SH, RYU SW, CHOI H, et al. Manufacturing Therapeutic Exosomes: from Bench to Industry. Mol Cells. 2022;45(5):284-290. [69] LIN BQ, LEI YM, WANG JX, et al. Microfluidic-Based Exosome Analysis for Liquid Biopsy. Small Methods. 2021;5(3):e2001131. [70] NOLAN JP, JONES JC. Detection of platelet vesicles by flow cytometry. Platelets. 2017; 28(3):256-262. [71] GUPTA A, KASHTE S, GUPTA M, et al. Mesenchymal stem cells and exosome therapy for COVID-19: current status and future perspective. Human Cell. 2020;33(4): 907-918. [72] NATASHA G, GUNDOGAN B, TAN A, et al. Exosomes as Immunotheranostic Nanoparticles. Clin Ther. 2014;36(6):820-829. [73] SMITH JA, DANIEL R. Human vaginal fluid contains exosomes that have an inhibitory effect on an early step of the HIV-1 life cycle. Aids. 2016;30(17):2611-2616. [74] LEE JH, PARK J, LEE JW. Therapeutic use of mesenchymal stem cell-derived extracellular vesicles in acute lung injury. Transfusion. 2019;59:876-883. [75] ZHAO AG, SHAH KR, CROMER B, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles and Their Therapeutic Potential. Stem Cells Int. 2020;2020:10. [76] YI KH, WINAYANUWATTIKUN W, KIM SY, et al. Skin boosters: Definitions and varied classifications. Skin Res Technol. 2024;30(3):e13627. [77] ZHOU Y, WEN LL, LI YF, et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen Res. 2022; 17(1):194-202. [78] KIM S, LEE S K, KIM H, et al. Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. Int J Mol Sci. 2018; 19(10):16. [79] MA T, FU B C, YANG X, et al. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120(6):10847-10854. [80] HU P, CHIARINI A, WU J, et al. Exosomes of adult human fibroblasts cultured on 3D silk fibroin nonwovens intensely stimulate neoangiogenesis. Burns Trauma. 2021;9: tkab003. [81] SUN CK, CHEN CH, CHANG CL, et al. Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Transl Res. 2017;9(4):1543-1560. [82] KWON HH, YANG SH, LEE J, et al. Combination Treatment with Human Adipose Tissue Stem Cell-derived Exosomes and Fractional CO2 Laser for Acne Scars: A 12-week Prospective, Double-blind, Randomized, Split-face Study. Acta Derm Venereol. 2020;100(18):adv00310. [83] BURKE J, HUNTER M, KOLHE R, et al. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin Transl Med. 2016;5(1):27. [84] HU HX, DONG LL, BU ZH, et al. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J Extracell Vesicles. 2020;9(1):1778883. [85] JIANG SP, TIAN GZ, YANG Z, et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact Mater. 2021;6(9): 2711-2728. [86] REN SH, LIN YY, LIU WY, et al. MSC-Exos: Important active factor of bone regeneration. Front Bioeng Biotechnol. 2023:11:1136453. [87] 刘闯,谭龙旺,周禾山,等.脂肪间充质干细胞外泌体治疗创伤性中枢神经系统损伤[J].中国组织工程研究,2023,27(19): 3061-3069. [88] DING M, SHEN Y, WANG P, et al. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer’s Disease. Neurochem Res. 2018; 43(11):2165-2177. [89] LEE M, BAN JJ, YANG S, et al. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1691: 87-93. [90] SONG JY, SUN TT, TANG Z, et al. Exosomes derived from smooth muscle cells ameliorate diabetes-induced erectile dysfunction by inhibiting fibrosis and modulating the NO/cGMP pathway. J Cell Mol Med. 2020;24(22): 13289-13302. [91] MITSIALIS V, WALL S, LIU P, et al. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn’s Disease. Gastroenterology. 2020; 159(2):591-608.e10. [92] NISHIDA A, INOUE R, INATOMI O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018; 11(1):1-10. [93] LI YJ, XU QW, XU CH, et al. MSC Promotes the Secretion of Exosomal miR-34a-5p and Improve Intestinal Barrier Function Through METTL3-Mediated Pre-miR-34A m6A Modification. Mol Neurobiol. 2022;59(8):5222-5235. [94] WU XR, LAN P, WU XJ, et al. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019; 4(24):e131273. [95] WANG GY, JOEL MDM, YUAN JT, et al. Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease by inhibiting ERK phosphorylation in neutrophils. Inflammopharmacology. 2020;28(2):603-616. [96] CAI X, ZHANG ZY, YUAN JT, et al. hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res Ther. 2021;12(1):416. [97] KALOGERIS T, BAINES CP, KRENZ M, et al. Ischemia/Reperfusion. Compr Physiol. 2016; 7(1):113-170. [98] REN Y, WU Y, HE WS, et al. Exosomes secreted from bone marrow mesenchymal stem cells suppress cardiomyocyte hypertrophy through Hippo-YAP pathway in heart failure. Genet Mol Biol. 2023;46(1):e20220221. [99] WANG ZC, GAO D, WANG SJ, et al. Exosomal microRNA-1246 from human umbilical cord mesenchymal stem cells potentiates myocardial angiogenesis in chronic heart failure. Cell Biol Int. 2021;45(11):2211-2225. [100] LIU L, JIN X, HU CF, et al. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell Physiol Biochem. 2017;43(1):52-68. [101] LIN ZJ, WU YL, XU YT, et al. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21(1):179. [102] LOU GH, SONG XL, YANG F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015:8:122. [103] LOU GH, CHEN L, XIA CX, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39(1):4. [104] SHARIF S, GHAHREMANI MH, SOLEIMANI M. Delivery of Exogenous miR-124 to Glioblastoma Multiform Cells by Wharton’s Jelly Mesenchymal Stem Cells Decreases Cell Proliferation and Migration, and Confers Chemosensitivity. Stem Cell Rev Rep. 2018;14(2):236-246. [105] CHEN HY, SUN T, JIANG C. Extracellular vesicle-based macromolecule delivery systems in cancer immunotherapy. J Control Release. 2022:348:572-589. [106] YANG Q, LI SS, OU HB, et al. Exosome-based delivery strategies for tumor therapy: an update on modification, loading, and clinical application. J Nanobiotechnology. 2024;22(1):41. [107] ZHAO M, LIU SY, WANG CS, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano. 2021;15(1): 1519-1538. [108] 王双敏,汪显耀,何志旭.工程化间充质干细胞来源外泌体在靶向递送抗肿瘤药物中的应用与问题[J].中国组织工程研究,2025, 29(23):4975-4983. [109] DEHNAVI S, KHODADADI A, ASADIRAD A, et al. Loading Ovalbumin into Mesenchymal Stem Cell-Derived Exosomes as a Nanoscale Carrier with Immunomodulatory Potential for Allergen-Specific Immunotherapy. Rep Biochem Mol Biol. 2023;11(4):626-634. |
| [1] | 杨 肖, 白月辉, 赵甜甜, 王东昊, 赵 琛, 袁 硕. 颞下颌关节骨关节炎软骨退变:机制及再生的挑战[J]. 中国组织工程研究, 2026, 30(4): 926-935. |
| [2] | 余诗宇, 俞苏桐, 徐 杨, 镇祥燕, 韩凤选. 组织工程治疗策略在口腔黏膜下纤维化中的研究与应用进展[J]. 中国组织工程研究, 2026, 30(4): 936-948. |
| [3] | 王宇航, 张 涵, 张超晶, 寇绪容, 井桐桐, 林日梅, 刘鑫宇, 娄石磊, 阎 慧, 孙 聪. 姜黄素提取及姜黄素纳米粒的制备及优化[J]. 中国组织工程研究, 2026, 30(2): 362-374. |
| [4] | 谭凤怡, 谢嘉敏, 潘振锋, 张新旭, 郑泽态, 曾祉莹, 周艳芳. 胶原蛋白联合微针治疗皮肤光老化的作用及机制[J]. 中国组织工程研究, 2026, 30(2): 451-458. |
| [5] | 于漫亚, 崔 兴. 骨髓微环境中不同细胞对多发性骨髓瘤骨病外泌体环状RNA的贡献及相互作用[J]. 中国组织工程研究, 2026, 30(1): 101-110. |
| [6] | 陈启衡, 翁土军, 彭 江. 二甲基氧化甘氨酸对人骨髓间充质干细胞成骨、成脂分化及线粒体自噬的影响[J]. 中国组织工程研究, 2026, 30(1): 50-57. |
| [7] | 袁为远, 雷秦袆, 李秀琪, 卢铁柱, 傅子文, 梁志丽, 季韶洋, 李一佳, 任 宇. 脂肪来源间充质干细胞及外泌体对地塞米松诱导肌肉减少症小鼠的治疗作用[J]. 中国组织工程研究, 2026, 30(1): 58-67. |
| [8] | 马文静, 张晋豫, 姜明霞, 修冰水, 白 睿, 刘玉含, 陈旭义, 袁增强, 刘志强. 无支架三维人脐带间充质干细胞分泌组修复小鼠皮肤损伤[J]. 中国组织工程研究, 2026, 30(1): 68-77. |
| [9] | 牟彦郦, 胡安春, 须文驰, 陈盼盼, 陈 浩, 赵淑云, 黄官友, 陈小娟. 人脐血富血小板血浆、单个核细胞及间充质干细胞修复大鼠薄型子宫内膜[J]. 中国组织工程研究, 2026, 30(1): 78-92. |
| [10] | 吴芷菁, 李加利, 张佳昕, 王唐蓉, 郑煜洲, 孙梓暄. α-酮戊二酸工程化小细胞外囊泡延缓皮肤衰老[J]. 中国组织工程研究, 2026, 30(1): 120-129. |
| [11] | 徐海超, 罗丽花, 潘乙怀. 牙髓干细胞及衍生产物在牙髓再生中的应用与进展[J]. 中国组织工程研究, 2026, 30(1): 153-162. |
| [12] | 张钊伟, 陈欧子乐, 白明茹, 汪成林. 牙源性间充质干细胞分泌生物活性物质用于骨修复的治疗潜力[J]. 中国组织工程研究, 2026, 30(1): 163-174. |
| [13] | 刘 念, 董昕玥, 王菘芃, 徐英江, 张晓明. 干细胞外泌体和生物材料辅助外泌体修复骨缺损[J]. 中国组织工程研究, 2026, 30(1): 175-183. |
| [14] | 吕茹月, 顾路路, 刘 茜, 周思仪, 李贝贝, 薛乐天, 孙 鹏. 外泌体分泌调控机制及在生物医学中的应用前景[J]. 中国组织工程研究, 2026, 30(1): 184-193. |
| [15] | 罗文彬, 李若云, 潘超凡, 罗长江. 工程化外泌体修复组织损伤:应用潜力及优异的生物稳定性和靶向特异性[J]. 中国组织工程研究, 2026, 30(1): 204-217. |
外泌体广泛存在于各种体液及细胞培养基中,与微囊泡、凋亡小体及蛋白质聚集体等具有相似的物理化学性质[4],因此,一般的分离技术很难将其分离。目前常用的外泌体分离方法有超速离心法、超滤法、尺寸排阻色谱法、免疫捕获法、聚合物沉淀法以及微流控技术等。虽然外泌体分离方法不断发展,但大多数分离技术往往不能同时保证外泌体高纯度和高产率制备要求,且存在操作步骤繁杂、分离时间长、破坏膜状结构等问题。为此,该文综述现有外泌体分离纯化技术和鉴定方法,并阐述间充质干细胞外泌体在临床应用中的挑战和未来发展方向。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 由第一作者在2024年6-9月进行检索。
1.1.2 检索文献时限 各数据库2024年9月之前发表的文献。
1.1.3 检索数据库 中国知网、PubMed和Web of Science数据库。
1.1.4 检索途径 主题词检索、关键词检索、摘要检索等。
1.1.5 检索词 中文检索词为“外泌体,间充质干细胞,分离纯化,表征,临床应用”,英文检索词为“exosome,extracellular vesicles,mesenchymal stem cells,isolation,characterization,
application”。
1.1.6 检索文献类型 研究原著和综述。
1.1.7 检索策略 中英文数据库检索策略,见图1。
1.1.8 检索文献量 初步检索到英文文献1 655篇,中文文献137篇。
1.2 入选标准
1.2.1 纳入标准 ①与间充质干细胞外泌体的分离纯化、标准或临床应用有关的文献;②具有创新性、权威性、可靠性的文献;③相似领域研究的文献选取近3年发表的文献。
1.2.2 排除标准 ①研究内容与间充质干细胞外泌体无关或相关性较差的文献;②相同类型的研究;③年代较为久远的文献。
1.3 文献质量评估和数据的提取 初步检索得到相关文献1 792篇。通过纳入标准和排除标准进行筛选,最终确定源于中国知网的中文文献2篇、源于PubMed 数据库和 Web of Science 数据库英文文献107篇进行归纳分析。文献筛选流程图见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
目前外泌体分离纯化所面临的技术障碍主要分为以下几点:①外泌体自身结构复杂,与脂蛋白、病毒等细胞外囊泡物理化学和生化特性重叠,本身异质性导致难有通用分离技术。目前常用的分离方法很难有效区分外泌体及与其物理性质相似的杂质,且分离规模小、成本高。②不同纯化分离方法因原理不同获得的外泌体难以维持同样的纯度和浓度,批次间差异大,稳定性不高,导致无法精确表征外泌体的数量和质量。因此,开发高效大规模分离纯化外泌体的工艺仍然是目前亟需解决的问题。③目前外泌体的生产大多基于实验室小规模培养,无法大规模生产满足临床标准的稳定外泌体。此外,外泌体的膜结构不稳定,在储存、运输及外泌体的使用过程中极易降解,影响疾病的治疗效果。
3.2 作者综述区别于他人他篇的特点 目前已有文献综述介绍了外泌体的分离、表征及相关应用,但此文在阐述间充质干细胞外泌体于不同疾病领域临床应用领域的基础上,结合外泌体的分离和表征技术,尤其是源于细胞培养基的外泌体的分离技术,为间充质干细胞外泌体的规模化生产和临床转化提出见解。此外,文章对间充质干细胞外泌体的临床应用进行广泛介绍,并将生产、鉴定和应用结合,引入最新研究成果,为不同专业的研究者们提供对间充质干细胞外泌体研究进展的全面认知。
3.3 综述的局限性 该综述总结了间充质干细胞外泌体的分离、表征及应用,但所依据的大部分为细胞和动物实验,缺少大规模临床数据研究。此外,由于篇幅限制,对于间充质干细胞外泌体临床应用的介绍较为简单,且相关机制的探索也不够全面。
3.4 综述的重要意义 间充质干细胞因强大的自我更新和分化能力在临床医学上得到广泛应用,而其分泌的外泌体作为间充质干细胞的主要作用成分由于免疫原性更低、靶向效果更强等优势而具有远大的应用前景。文章综述了目前常用的外泌体分离技术与鉴定方法,比较其优缺点,分析目前的生产困境与未来的发展方向,帮助研究者迅速了解外泌体生产领域的相关研究成果。在此基础上,进一步阐述了间充质干细胞外泌体在不同疾病领域的临床应用及有关作用机制,深入探讨间充质干细胞外泌体目前的应用前景及临床转化的障碍,为未来研究者对该领域的探索提供了切实可靠的理论依据与实践基础。
3.5 展望 面对间充质干细胞外泌体难以规模化生产的问题,在综合考虑外泌体获取的纯度和产量、外泌体的稳定性与生物活性,以及分离纯化方案所需时间与成本等因素的基础上,可以从以下方面进行技术研究:①研究外泌体分泌或形成的细胞培养工艺。从细胞培养开始保持外泌体分泌过程的稳定,建立动态监测培养环境条件与代谢指标的三维培养间充质干细胞的工艺,保证批次间相对数量和质量的稳定。②开发高效和规模化外泌体分离纯化技术。外泌体应用的关键是最终产物的产量,通过开发高产量外泌体制备方法的同时追求外泌体更高的纯度。③外泌体分离方法应兼顾下游的分析与应用,在分离方案开发的过程中尽可能考虑到后续临床转化的需求,如外泌体的收集、储存和制剂等技术。
总之,随着分离技术的发展,间充质干细胞外泌体发展的困境终将被克服,未来有望在现有技术的基础上尝试不同的发展方向,以推动外泌体分离鉴定方案的发展,从而促进间充质干细胞外泌体的临床转化进程。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
间充质干细胞:是一种具有自我更新能力和多向分化潜能的干细胞,存在于脂肪、脐带和骨髓等多种组织和器官中,对机体修复和免疫调节有重要作用,在组织工程和再生医学中有广泛的应用前景。
外泌体:是一种直径在30-150 nm的细胞外囊泡,由细胞在正常或异常状态下分泌,能够传递蛋白质、核酸和脂质等生物活性物质至靶细胞以介导细胞间通讯,从而调节细胞与组织的功能。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
面对间充质干细胞外泌体难以规模化生产的问题,在综合考虑外泌体获取的纯度和产量、外泌体的稳定性与生物活性,以及分离纯化方案所需时间与成本等因素的基础上,可以从以下方面技术研究:①研究外泌体分泌或形成的细胞培养工艺。从细胞培养开始保持外泌体分泌过程的稳定,建立动态监测培养环境条件与代谢指标的三维培养间充质干细胞的工艺,保证批次间相对数量和质量的稳定。②开发高效和规模化外泌体分离纯化技术。外泌体应用的关键是最终产物的产量,通过开发高产量外泌体制备方法的同时追求外泌体更高的纯度。③外泌体分离方法应兼顾下游的分析与应用,在分离方案开发的过程中尽可能考虑到后续临床转化的需求,如外泌体的收集、储存和制剂等技术。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||