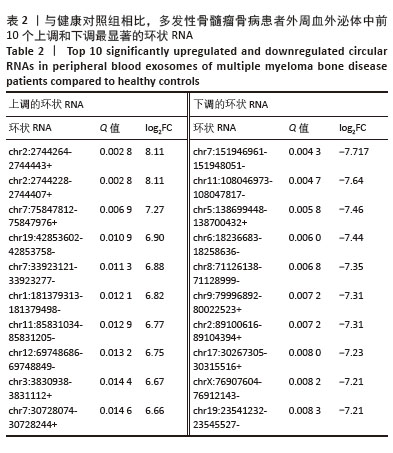
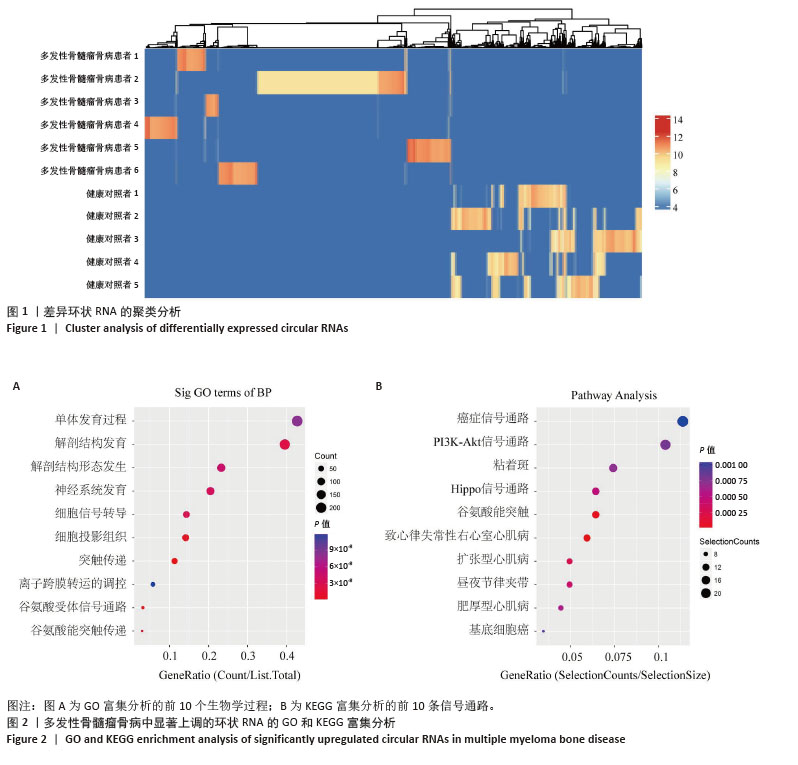
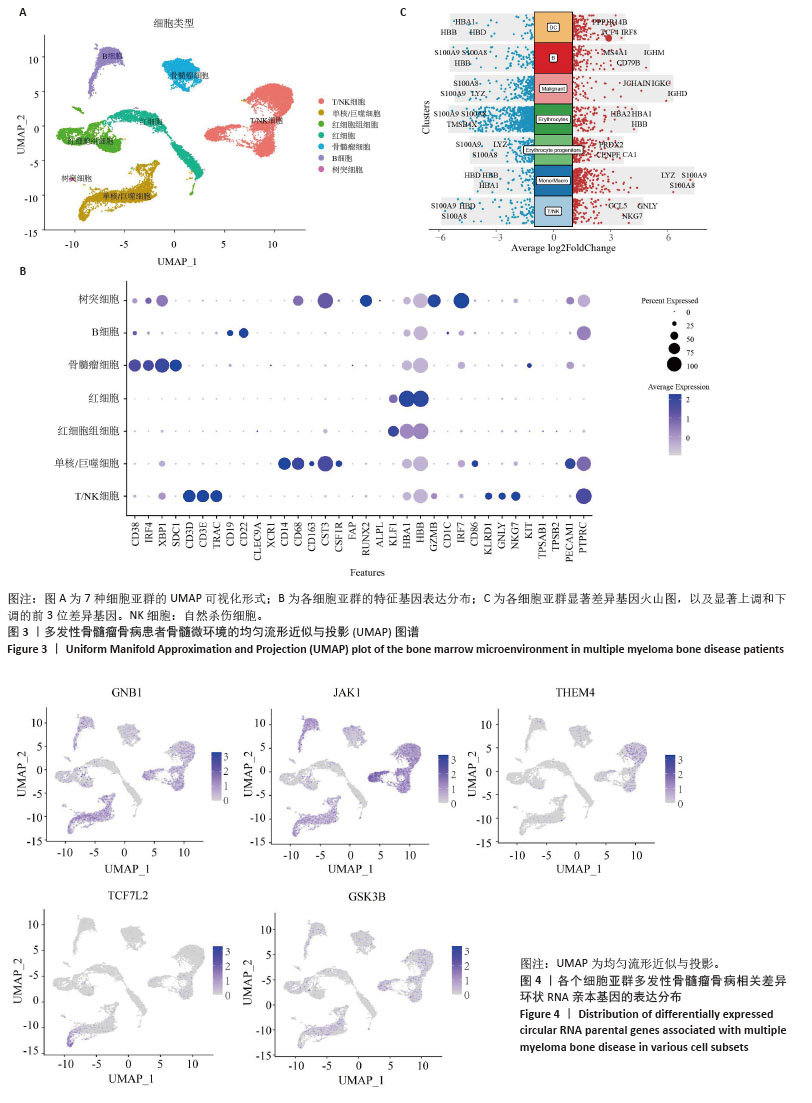
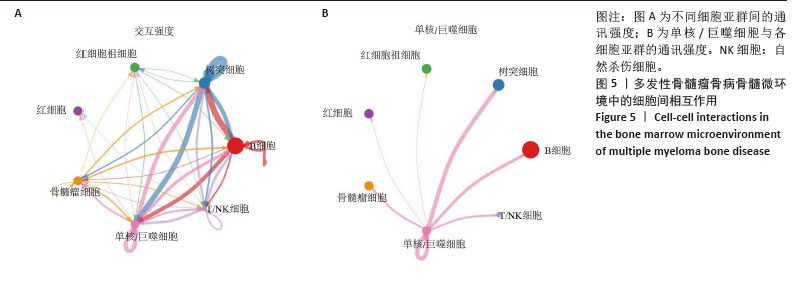
[1] MALARD F, NERI P, BAHLIS NJ, et al. Multiple myeloma. Nat Rev Dis Primers. 2024;10(1):45.
[2] MUKKAMALLA SKR, MALIPEDDI D. Myeloma Bone Disease: A Comprehensive Review. Int J Mol Sci. 2021;22(12):6208.
[3] YU D, LI Y, WANG M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56.
[4] BERNSTEIN ZS, KIM EB, RAJE N. Bone Disease in Multiple Myeloma: Biologic and Clinical Implications. Cells. 2022;11(15): 2308.
[5] MENU E, VANDERKERKEN K. Exosomes in multiple myeloma: from bench to bedside. Blood. 2022;140(23):2429-2442.
[6] LIU R, ZHONG Y, CHEN R, et al. m6A reader hnRNPA2B1 drives multiple myeloma osteolytic bone disease. Theranostics. 2022; 12(18):7760-7774.
[7] LI B, XU H, HAN H, et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene. 2018;37(41): 5508-5519.
[8] UNTI MJ, JAFFREY SR. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem Biol. 2024; 31(1):163-176.e5.
[9] ZHANG F, JIANG J, QIAN H, et al. Exosomal circRNA: emerging insights into cancer progression and clinical application potential. J Hematol Oncol. 2023;16(1):67.
[10] ARAN D, LOONEY AP, LIU L, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20(2):163-172.
[11] KIKUCHI H, AMOFA E, MCENERY M, et al. Inhibition of PI3K Class IA Kinases Using GDC-0941 Overcomes Cytoprotection of Multiple Myeloma Cells in the Osteoclastic Bone Marrow Microenvironment Enhancing the Efficacy of Current Clinical Therapeutics. Cancers (Basel). 2023;15(2):462.
[12] LI H, ZHANG Y, MOU X, et al. Interference with PLA2G16 promotes cell cycle arrest and apoptosis and inhibits the reprogramming of glucose metabolism in multiple myeloma cells by modulating the Hippo/YAP signaling pathway. Anticancer Drugs. 2024;35(10):902-911.
[13] TSUBAKI M, KATO C, MANNO M, et al. Macrophage inflammatory protein-1alpha (MIP-1alpha) enhances a receptor activator of nuclear factor kappaB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol Cell Biochem. 2007;304(1-2):53-60.
[14] PAN JX, XIONG L, ZHAO K, et al. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Res. 2018;6:18.
[15] YANG W, HAN W, QIN A, et al. The emerging role of Hippo signaling pathway in regulating osteoclast formation. J Cell Physiol. 2018;233(6): 4606-4617.
[16] MIAOMIAO S, XIAOQIAN W, YUWEI S, et al. Cancer-associated fibroblast-derived exosome microRNA-21 promotes angiogenesis in multiple myeloma. Sci Rep. 2023;13(1):9671.
[17] MIZUHARA K, SHIMURA Y, TSUKAMOTO T, et al. Tumour-derived exosomes promote the induction of monocytic myeloid-derived suppressor cells from peripheral blood mononuclear cells by delivering miR-106a-5p and miR-146a-5p in multiple myeloma. Br J Haematol. 2023;203(3):426-438.
[18] WANG Z, HE J, BACH DH, et al. Induction of m6A methylation in adipocyte exosomal LncRNAs mediates myeloma drug resistance. J Exp Clin Cancer Res. 2022;41(1):4.
[19] YU M, JI L, LI S, et al. Exosomal circ-CACNG2 promotes cardiomyocyte apoptosis in multiple myeloma via modulating miR-197-3p/caspase3 axis. Exp Cell Res. 2022;417(2):113229.
[20] ALIMOHAMMADI M, RAHIMZADEH P, KHORRAMI R, et al. A comprehensive review of the PTEN/PI3K/Akt axis in multiple myeloma: From molecular interactions to potential therapeutic targets. Pathol Res Pract. 2024;260:155401.
[21] HEINEMANN L, MÖLLERS KM, AHMED HMM, et al. Inhibiting PI3K-AKT-mTOR Signaling in Multiple Myeloma-Associated Mesenchymal Stem Cells Impedes the Proliferation of Multiple Myeloma Cells. Front Oncol. 2022;12:874325.
[22] KYRIAZOGLOU A, NTANASIS-STATHOPOULOS I, TERPOS E, et al. Emerging Insights Into the Role of the Hippo Pathway in Multiple Myeloma and Associated Bone Disease. Clin Lymphoma Myeloma Leuk. 2020;20(2):57-62.
[23] YANG J, ZHANG X, WANG J, et al. Anti beta2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts. Blood. 2007;110(8):3028-3035.
[24] MENG B, WU D, CHENG Y, et al. Interleukin-20 differentially regulates bone mesenchymal stem cell activities in RANKL-induced osteoclastogenesis through the OPG/RANKL/RANK axis and the NF-κB, MAPK and AKT signalling pathways. Scand J Immunol. 2020;91(5): e12874.
[25] ZHANG Z, ZHANG X, ZHAO D, et al. TGF‑β1 promotes the osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1 signalling pathway. Mol Med Rep. 2019;19(5):3505-3518.
[26] MARTIN SK, GAN ZY, FITTER S, et al. The effect of the PI3K inhibitor BKM120 on tumour growth and osteolytic bone disease in multiple myeloma. Leuk Res. 2015;39(3):380-387.
[27] SEO E, BASU-ROY U, GUNARATNE PH, et al. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3(6):2075-2087.
[28] MATSUMOTO Y, LA ROSE J, KENT OA, et al. Reciprocal stabilization of ABL and TAZ regulates osteoblastogenesis through transcription factor RUNX2. J Clin Invest. 2016;126(12):4482-4496.
[29] PARK H, NOH AL, KANG JH, et al. Peroxiredoxin II negatively regulates lipopolysaccharide-induced osteoclast formation and bone loss via JNK and STAT3. Antioxid Redox Signal. 2015;22(1):63-77.
[30] LU J, YE C, HUANG Y, et al. Corilagin suppresses RANKL-induced osteoclastogenesis and inhibits oestrogen deficiency-induced bone loss via the NF-κB and PI3K/AKT signalling pathways. J Cell Mol Med. 2020;24(18):10444-10457.
[31] HUANG F, WONG P, LI J, et al. Osteoimmunology: The correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J Cell Mol Med. 2022;26(13):3591-3597.
[32] WU LZ, DUAN DM, LIU YF, et al. Nicotine favors osteoclastogenesis in human periodontal ligament cells co-cultured with CD4(+) T cells by upregulating IL-1β. Int J Mol Med. 2013;31(4):938-942.
[33] NAGAI S, KUREBAYASHI Y, KOYASU S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann N Y Acad Sci. 2013;1280: 30-34.
[34] FU M, HU Y, LAN T, et al. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. 2022;7(1):376.
[35] SÖDERSTRÖM K, STEIN E, COLMENERO P, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci U S A. 2010;107(29):13028-13033.
[36] FENG P, LUO L, YANG Q, et al. Hippo kinases Mst1 and Mst2 maintain NK cell homeostasis by orchestrating metabolic state and transcriptional activity. Cell Death Dis. 2024;15(6):430.
[37] ALI AK, NANDAGOPAL N, LEE SH. IL-15-PI3K-AKT-mTOR: A Critical Pathway in the Life Journey of Natural Killer Cells. Front Immunol. 2015;6:355.
[38] CHEN Y, WANG H, NI Q, et al. B-Cell-Derived TGF-β1 Inhibits Osteogenesis and Contributes to Bone Loss in Periodontitis. J Dent Res. 2023;102(7):767-776.
[39] FISCHER V, HAFFNER-LUNTZER M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14-21.
[40] GRČEVIĆ D, SANJAY A, LORENZO J. Interactions of B-lymphocytes and bone cells in health and disease. Bone. 2023;168:116296.
[41] HODSON DJ, TURNER M. The role of PI3K signalling in the B cell response to antigen. Adv Exp Med Biol. 2009;633:43-53.
[42] ZHENG L, GAO J, JIN K, et al. Macrophage migration inhibitory factor (MIF) inhibitor 4-IPP suppresses osteoclast formation and promotes osteoblast differentiation through the inhibition of the NF-κB signaling pathway. FASEB J. 2019;33(6):7667-7683.
[43] HE J, ZHENG L, LI X, et al. Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss. J Adv Res. 2023;53:235-248.
|