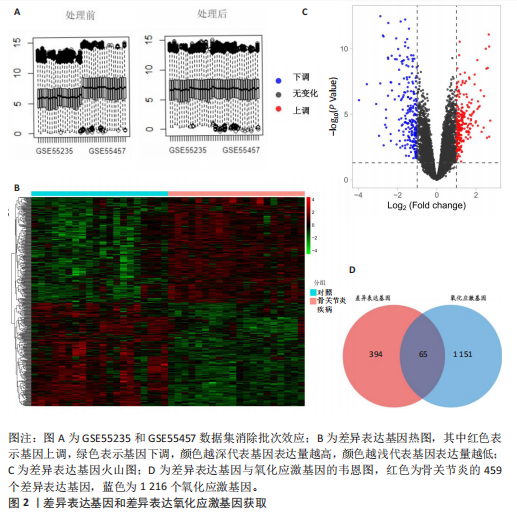
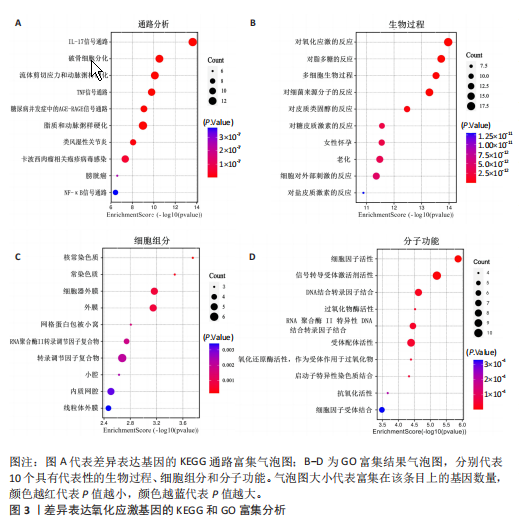
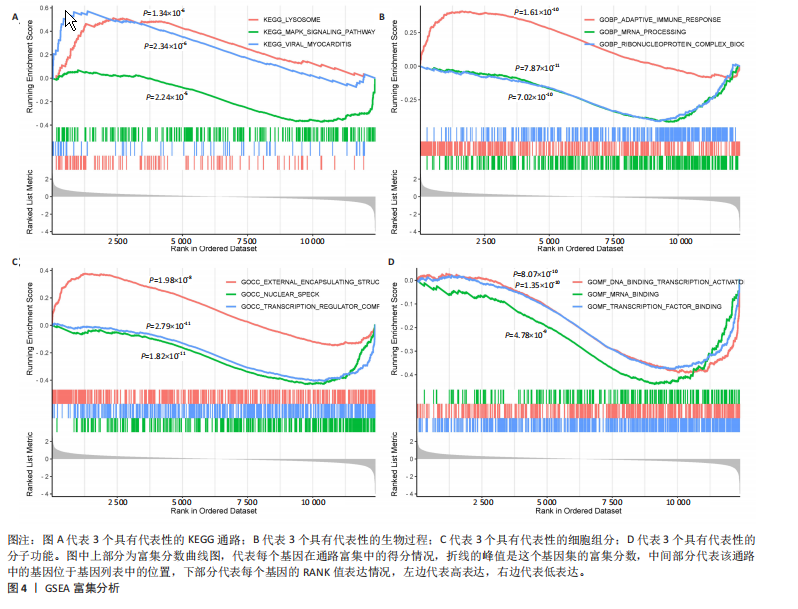
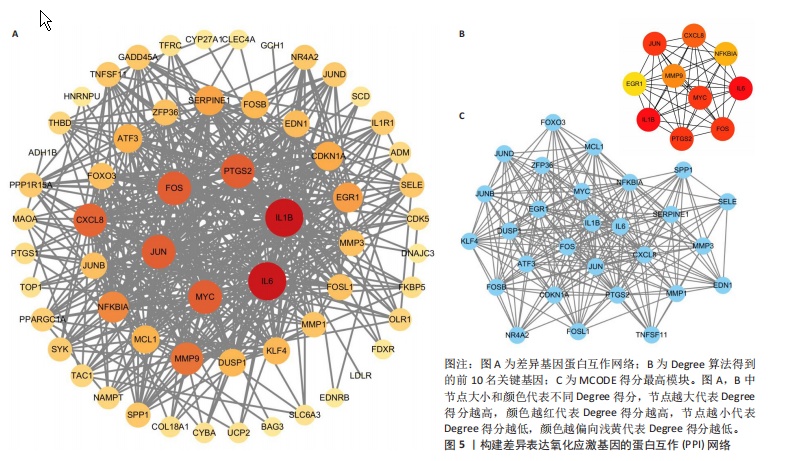
[1] HASEEB A, HAQQI TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013; 146(3):185-196.
[2] MOLNAR V, MATIŠIĆ V, KODVANJ I, et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int J Mol Sci. 2021;22(17):9208.
[3] SIDDIQ MAB, CLEGG D, JANSEN TL, et al. Emerging and New Treatment Options for Knee Osteoarthritis. Curr Rheumatol Rev. 2022;18(1):20-32.
[4] ZHU X, SANG L, WU D, et al. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2018;13(1):170.
[5] MARTEL-PELLETIER J, BARR AJ, CICUTTINI FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072.
[6] ASTRIKE-DAVIS EM, CORYELL P, LOESER RF. Targeting cellular senescence as a novel treatment for osteoarthritis. Curr Opin Pharmacol. 2022;64:102213.
[7] FOIS AG, PALIOGIANNIS P, SOTGIA S, et al. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir Res. 2018;19(1):51.
[8] DAENEN K, ANDRIES A, MEKAHLI D, et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34(6):975-991.
[9] LIU L, LUO P, YANG M, et al. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front Mol Biosci. 2022;9:1001212.
[10] BLANCO FJ, VALDES AM, REGO-PÉREZ I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat Rev Rheumatol. 2018;14(6):327-340.
[11] RAJAGOPALAN S, MENG XP, RAMASAMY S, et al. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98(11):2572-2579.
[12] PETERSEN SV, OURY TD, OSTERGAARD L, et al. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279(14):13705-13710.
[13] SCOTT JL, GABRIELIDES C, DAVIDSON RK, et al.
Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010;69(8):1502-1510.
[14] NEDUNCHEZHIYAN U, VARUGHESE I, SUN AR, et al. Obesity, Inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022;13:907750.
[15] LINDBLAD S, HEDFORS E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987;30(10):1081-1088.
[16] SAITO I, KOSHINO T, NAKASHIMA K, et al. Increased cellular infiltrate in inflammatory synovia of osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(2): 156-162.
[17] LUAN T, YANG X, KUANG G, et al. Identification and Analysis of Neutrophil Extracellular Trap-Related Genes in Osteoarthritis by Bioinformatics and Experimental Verification. J Inflamm Res. 2023;16:3837-3852.
[18] DUAN ZX, LI YS, TU C, et al. Identification of a potential gene target for osteoarthritis based on bioinformatics analyses. J Orthop Surg Res. 2020;15(1):228.
[19] 徐义峰,柯诗文,李可可,等.氧化应激和免疫浸润在特发性肺纤维化中的作用及中药防治研究[J/OL].中国免疫学杂志,1-10[2024-02-16]. https://link.cnki.net/urlid/22.1126.R.20231208.1514.004
[20] LIBERZON A, BIRGER C, THORVALDSDÓTTIR H, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417-425.
[21] GU X, LAI D, LIU S, et al. Hub Genes, Diagnostic Model, and Predicted Drugs Related to Iron Metabolism in Alzheimer’s Disease. Front Aging Neurosci. 2022;14:949083.
[22] KULESHOV MV, JONES MR, ROUILLARD AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90-97.
[23] AGARWAL V, BELL GW, NAM JW, et al. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4: e05005.
[24] YOO M, SHIN J, KIM J, et al. DSigDB: drug signatures database for gene set analysis. Bioinformatics. 2015;31(18):3069-3071.
[25] YUNUS MHM, NORDIN A, KAMAL H. Pathophysiological Perspective of Osteoarthritis. Medicina (Kaunas). 2020; 56(11):614.
[26] SACITHARAN PK. Ageing and Osteoarthritis. Subcell Biochem. 2019;91:123-159.
[27] HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298-300.
[28] BOLDUC JA, COLLINS JA, LOESER RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73-82.
[29] KIMBALL JS, JOHNSON JP, CARLSON DA. Oxidative Stress and Osteoporosis. J Bone Joint Surg Am. 2021;103(15):1451-1461.
[30] KAPOOR M, MARTEL-PELLETIER J, LAJEUNESSE D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33-42.
[31] STANNUS O, JONES G, CICUTTINI F, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441-1447.
[32] GREENE MA, LOESER RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11): 1966-1971.
[33] XIAO J, ZHANG P, CAI FL, et al. IL-17 in osteoarthritis: A narrative review. Open Life Sci. 2023;18(1):20220747.
[34] ROBERT M, MIOSSEC P. IL-17 in Rheumatoid Arthritis and Precision Medicine: From Synovitis Expression to Circulating Bioactive Levels. Front Med (Lausanne). 2019;5:364.
[35] TSUKAZAKI H, KAITO T. The Role of the IL-23/IL-17 Pathway in the Pathogenesis of Spondyloarthritis. Int J Mol Sci. 2020; 21(17):6401.
[36] BLAUVELT A, CHIRICOZZI A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379-390.
[37] ZHANG X, YUAN Y, PAN Z, et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: A meta-analysis. Clin Chim Acta. 2019;496: 76-83.
[38] MIMPEN JY, BALDWIN MJ, CRIBBS AP, et al. Interleukin-17A Causes Osteoarthritis-Like Transcriptional Changes in Human Osteoarthritis-Derived Chondrocytes and Synovial Fibroblasts In Vitro. Front Immunol. 2021;12:676173.
[39] WANG K, XU J, CAI J, et al. Serum levels of resistin and interleukin-17 are associated with increased cartilage defects and bone marrow lesions in patients with knee osteoarthritis. Mod Rheumatol. 2017; 27(2):339-344.
[40] 胡光亮,马振华,徐迈,等.骨关节炎患者TNF-α、IL-17、COMP、ADAMTS-7及mRNA表达水平研究[J].中国医学前沿杂志(电子版),2018,10(12):100-103.
[41] XIN R, XU Y, LONG D, et al. Mitochonic Acid-5 Inhibits Reactive Oxygen Species Production and Improves Human Chondrocyte Survival by Upregulating SIRT3-Mediated, Parkin-dependent Mitophagy. Front Pharmacol. 2022;13:911716.
[42] BENT R, MOLL L, GRABBE S, et al. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int J Mol Sci. 2018;19(8): 2155.
[43] DI FRANCESCO M, FRAGASSI A, PANNUZZO M, et al. Management of osteoarthritis: From drug molecules to nano/micromedicines. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022; 14(3):e1780.
[44] GONG Z, WANG Y, LI L, et al. Cardamonin alleviates chondrocytes inflammation and cartilage degradation of osteoarthritis by inhibiting ferroptosis via p53 pathway. Food Chem Toxicol. 2023;174:113644.
[45] XU W, ZHANG B, XI C, et al. Ferroptosis Plays a Role in Human Chondrocyte of Osteoarthritis Induced by IL-1β In Vitro. Cartilage. 2023;14(4):455-466.
[46] LIU L, ZHANG W, LIU T, et al. The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. 2023;62:102663.
[47] RUSSO RC, GARCIA CC, TEIXEIRA MM, et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10(5):593-619.
[48] BAGGIOLINI M. CXCL8 - The First Chemokine. Front Immunol. 2015;6:285.
[49] CAMBIER S, GOUWY M, PROOST P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol. 2023; 20(3):217-251.
[50] YANG P, TAN J, YUAN Z, et al. Expression profile of cytokines and chemokines in osteoarthritis patients: Proinflammatory roles for CXCL8 and CXCL11 to chondrocytes. Int Immunopharmacol. 2016;40:16-23.
[51] 杨鹏.骨关节炎患者细胞因子/趋化因子表达谱分析以及CXCL8和CXCL11对软骨细胞的调节作用[D].西安:第四军医大学,2018.
[52] GE X, SHI R, MA X. The secreted protein WNT5A regulates condylar chondrocyte proliferation, hypertrophy and migration. Arch Oral Biol. 2017;82:171-179.
[53] WU YH, LIU W, ZHANG L, et al. Effects of microRNA-24 targeting C-myc on apoptosis, proliferation, and cytokine expressions in chondrocytes of rats with osteoarthritis via MAPK signaling pathway. J Cell Biochem. 2018;119(10):7944-7958.
[54] ZOU J, LI XL, SHI ZM, et al. Effects of C-myc gene silencing on interleukin-1β-induced rat chondrocyte cell proliferation, apoptosis and cytokine expression. J Bone Miner Metab. 2018;36(3):286-296.
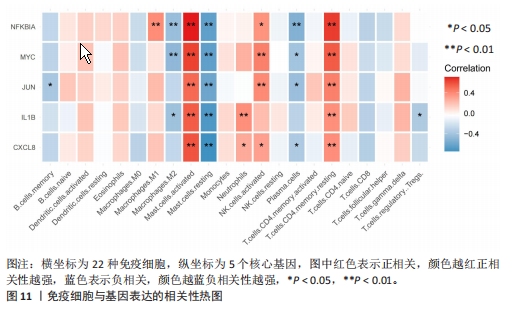
[55] TANG H, CHENG Z, MA W, et al. TLR10 and NFKBIA contributed to the risk of hip osteoarthritis: systematic evaluation based on Han Chinese population. Sci Rep. 2018;8(1):10243.
[56] CAI P, JIANG T, LI B, et al. Comparison of rheumatoid arthritis (RA) and osteoarthritis (OA) based on microarray profiles of human joint fibroblast-like synoviocytes. Cell Biochem Funct. 2019;37(1):31-41.
[57] LU H, HOU G, ZHANG Y, et al. c-Jun transactivates Puma gene expression to promote osteoarthritis. Mol Med Rep. 2014;9(5):1606-1612.
[58] YE Z, CHEN Y, ZHANG R, et al. c-Jun N-terminal kinase - c-Jun pathway transactivates Bim to promote osteoarthritis. Can J Physiol Pharmacol. 2014;92(2):132-139.
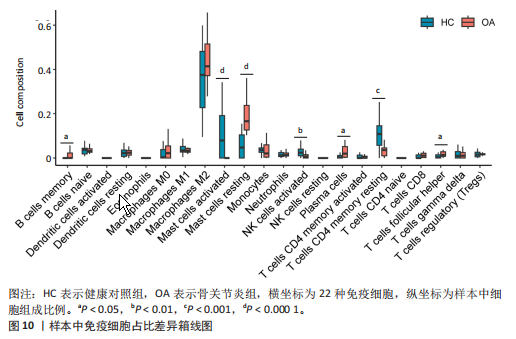
[59] DE LANGE-BROKAAR BJ, KLOPPENBURG M, ANDERSEN SN, et al. Characterization of synovial mast cells in knee osteoarthritis: association with clinical parameters. Osteoarthritis Cartilage. 2016;24(4):664-671.
[60] FARINELLI L, AQUILI A, MATTIOLI-BELMONTE M, et al. Synovial mast cells from knee and hip osteoarthritis: histological study and clinical correlations. J Exp Orthop. 2022;9(1):13.
[61] KULKARNI P, HARSULKAR A, MÄRTSON AG, et al. Mast Cells Differentiated in Synovial Fluid and Resident in Osteophytes Exalt the Inflammatory Pathology of Osteoarthritis. Int J Mol Sci. 2022;23(1):541.
[62] DOSS F, MENARD J, HAUSCHILD M, et al. Elevated IL-6 levels in the synovial fluid of osteoarthritis patients stem from plasma cells. Scand J Rheumatol. 2007;36(2): 136-139.
[63] QIAO D, GU C, WANG W, et al. Tumor-Originated Exosomal hsa-miR-3937 as a Minimally Invasive Early Biomarker for Liquid Biopsy of Colorectal Cancer. J Oncol. 2022;2022:6990955.
[64] SANCHEZ-RANGEL E, INZUCCHI SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586-1593.
[65] HE M, LU B, OPOKU M, et al. Metformin Prevents or Delays the Development and Progression of Osteoarthritis: New Insight and Mechanism of Action. Cells. 2022;11(19):3012.
[66] TERKELTAUB R, YANG B, LOTZ M, et al. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthritis Rheum. 2011;63(7):1928-1937.
[67] SONG Y, WU Z, ZHAO P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. Front Pharmacol. 2022;13:952560.
[68] PARK MJ, MOON SJ, BAEK JA, et al Metformin Augments Anti-Inflammatory and Chondroprotective Properties of Mesenchymal Stem Cells in Experimental Osteoarthritis. J Immunol. 2019;203(1): 127-136.
[69] WANG C, YAO Z, ZHANG Y, et al. Metformin Mitigates Cartilage Degradation by Activating AMPK/SIRT1-Mediated Autophagy in a Mouse Osteoarthritis Model. Front Pharmacol. 2020;11:1114.
[70] NA HS, KWON JY, LEE SY, et al. Metformin Attenuates Monosodium-Iodoacetate-Induced Osteoarthritis via Regulation of Pain Mediators and the Autophagy-Lysosomal Pathway. Cells. 2021;10(3):681.
[71] SCHADLER P, LOHBERGER B, STÜNDL N, et al. The Effect of Body Mass Index and Metformin on Matrix Gene Expression in Arthritic Primary Human Chondrocytes. Cartilage. 2021;13(2_suppl):1004S-1018S.
[72] BENIGNI G, DIMITROVA P, ANTONANGELI F, et al. CXCR3/CXCL10 Axis Regulates Neutrophil-NK Cell Cross-Talk Determining the Severity of Experimental Osteoarthritis. J Immunol. 2017;198(5):2115-2124.
[73] ROMERA-CÁRDENAS G, THOMAS LM, LOPEZ-COBO S, et al. Ionomycin Treatment Renders NK Cells Hyporesponsive. PLoS One. 2016;11(3):e0150998.
[74] GREGORI D, GIACOVELLI G, MINTO C, et al. Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. JAMA. 2018; 320(24):2564-2579. |