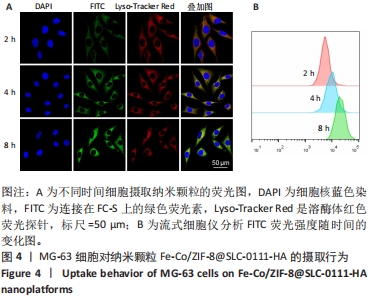
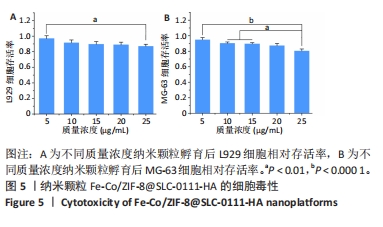
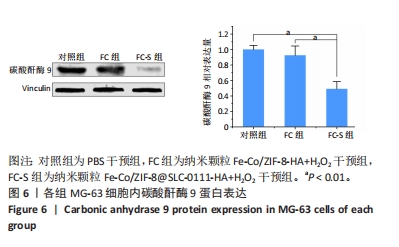
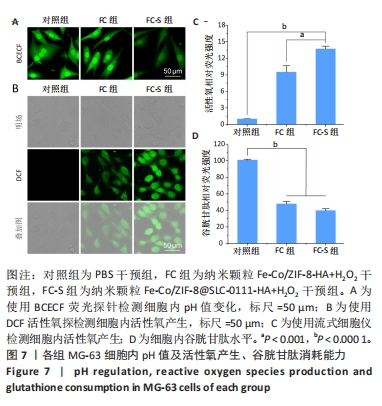
[1] KOO S, PARK OK, KIM J, et al. Enhanced Chemodynamic Therapy by Cu-Fe Peroxide Nanoparticles: Tumor Microenvironment-Mediated Synergistic Fenton Reaction. ACS Nano. 2022;16(2):2535-2545.
[2] ZHANG L, LI CX, WAN SS, et al. Nanocatalyst-Mediated Chemodynamic Tumor Therapy. Adv Healthc Mater. 2022;11(2):2101971.
[3] MOHAMMED DF, MADLOOL HA, FARIS M, et al. Harnessing Inorganic Nanomaterials for Chemodynamic Cancer Therapy. Nanomedicine. 2022;17(24):1891-1906.
[4] ZHAO P, LI H, BU W. A Forward Vision for Chemodynamic Therapy: Issues and Opportunities. Angew Chem Int Ed Engl. 2023;62(7): 202210415.
[5] GAO H, CAO Z, LIU H, et al. Multifunctional Nanomedicines-Enabled Chemodynamic-Synergized Multimodal Tumor Therapy via Fenton and Fenton-like Reactions. Theranostics. 2023;13(6):1974-2014.
[6] LI J, TIAN H, ZHU F, et al. Amorphous Ultra-Small Fe-Based Nanocluster Engineered and ICG Loaded Organo-Mesoporous Silica for GSH Depletion and Photothermal-Chemodynamic Synergistic Therapy. Adv Healthc Mater. 2022;11(21):2201986.
[7] ZHUANG J, WANG B, CHEN H, et al. Efficient NIR-II Type-I AIE Photosensitizer for Mitochondria-Targeted Photodynamic Therapy through Synergistic Apoptosis-Ferroptosis. ACS Nano. 2023;17(10): 9110-9125.
[8] ZHANG W, DU XF, LIU B, et al. Engineering Supramolecular Nanomedicine for Targeted Near Infrared-triggered Mitochondrial Dysfunction to Potentiate Cisplatin for Efficient Chemophototherapy. ACS Nano. 2022;16(1):1421-1435.
[9] GROELLY FJ, FAWKES M, DAGG RA, et al. Targeting DNA Damage Response Pathways in Cancer. Nat Rev Cancer. 2023;23(2):78-94.
[10] LI W, YIN S, SHEN Y, et al. Molecular Engineering of pH-Responsive NIR Oxazine Assemblies for Evoking Tumor Ferroptosis via Triggering Lysosomal Dysfunction. J Am Chem Soc. 2023;145(6):3736-3747.
[11] YANG Z, LUO Y, HU Y, et al. Photothermo-Promoted Nanocatalysis Combined with H2S-Mediated Respiration Inhibition for Efficient Cancer Therapy. Adv Funct Mater. 2021;31:2007991.
[12] ZUO W, CHEN W, LIU J, et al. Macrophage-Mimic Hollow Mesoporous Fe-Based Nanocatalysts for Self-Amplified Chemodynamic Therapy and Metastasis Inhibition via Tumor Microenvironment Remodeling. ACS Appl Mater Interfaces. 2022;14(4):5053-5065.
[13] WU C, LIU Z, CHEN Z, et al. A Nonferrous Ferroptosis-Like Strategy for Antioxidant Inhibition-Synergized Nanocatalytic Tumor Therapeutics. Sci Adv. 2021;7(39):eabj8833.
[14] MA Y, SU Z, ZHOU L, et al. Biodegradable Metal-Organic-Framework-Gated Organosilica for Tumor-Microenvironment-Unlocked Glutathione-Depletion-Enhanced Synergistic Therapy. Adv Mater. 2022;34(12):2107560.
[15] XU N, HU A, PU X, et al. Cu-Chelated Polydopamine Nanoparticles as a Photothermal Medium and “Immunogenic Cell Death” Inducer for combined tumor therapy. J Mater Chem B. 2022;10(16):3104-3118.
[16] DONG D, CHENG Z, WANG T, et al. Acid-Degradable Nanocomposite hydrogel and Glucose Oxidase Combination for Killing Bacterial with Photothermal Augmented Chemodynamic Therapy. Int J Biol Macromol. 2023;234:123745.
[17] YANG Z, ZHANG L, WEI J, et al. Tumor Acidity-Activatable Photothermal/Fenton Nanoagent for Synergistic Therapy. J Colloid Interface Sci. 2022; 612:355-366.
[18] JIA C, GUO Y, WU FG. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small. 2022;18(6):2103868.
[19] LIU Y, NIU R, DENG R, et al. Multi-enzyme Co-expressed Dual-Atom Nanozymes Induce Cascade Immunogenic Ferroptosis via Activating Interferon-γ and Targeting Arachidonic Acid Metabolism. J Am Chem Soc. 2023;145(16):8965-8978.
[20] LIU Z, LIU S, LIU B, et al. Fe(III)-Naphthazarin Metal–Phenolic Networks for Glutathione-Depleting Enhanced Ferroptosis–Apoptosis Combined Cancer Therapy. Small. 2023;19:2207825.
[21] MCDONALD PC, CHAFE SC, SUPURAN CT, et al. Cancer Therapeutic Targeting of Hypoxia Induced Carbonic Anhydrase IX: From Bench to Bedside. Cancers. 2022;14(14):3297.
[22] GAO D, CHEN T, CHEN S, et al. Targeting Hypoxic Tumors with Hybrid Nanobullets for Oxygen-Independent Synergistic Photothermal and Thermodynamic Therapy. Nanomicro Lett. 2021;13(1):99.
[23] QUEEN A, BHUTTO HN, YOUSUF M, et al. Carbonic anhydrase IX: A Tumor Acidification Switch in Heterogeneity and Chemokine Regulation. Semin Cancer Biol. 2022;86:899-913.
[24] DI FIORE A, SUPURAN CT, SCALONI A, et al. Post-translational Modifications in Tumor-Associated Carbonic Anhydrases. Amino Acids. 2022;54(4):543-558.
[25] CHAFE SC, VIZEACOUMAR FS, VENKATESWARAN G, et al. Genome-Wide Synthetic Lethal Screen Unveils Novel CAIX-NFS1/xCT Axis as a Targetable Vulnerability in Hypoxic Solid Tumors. Sci Adv. 2021;7(35): eabj0364.
[26] SARNELLA A, FERRARA Y, AULETTA L, et al. Inhibition of Carbonic Anhydrases IX/XII by SLC-0111 Boosts Cisplatin Effects in Hampering Head and Neck Squamous Carcinoma Cell Growth and Invasion. J Exp Clin Cancer Res. 2022;41(1):122.
[27] SU X, WANG WJ, CAO Q, et al. A Carbonic Anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer Evokes Pyroptosis for Enhanced Anti-Tumor Immunity. Angew Chem Int Ed Engl. 2022;61(8):202115800.
[28] WANG J, SUN Z, WANG S, et al. Biodegradable Ferrous Sulfide-Based Nanocomposites for Tumor Theranostics through Specific Intratumoral Acidosis-Induced Metabolic Symbiosis Disruption. J Am Chem Soc. 2022;144(43):19884-19895.
[29] LIANG S, LIAO G, ZHU W, et al. Manganese-Based Hollow Nanoplatforms for MR Imaging-Guided Cancer Therapies. Biomater Res. 2022;26(1):32.
[30] Sanchez-Uriel L, Bonet-Aleta J, Ibarra A, et al. Heterogeneous-Driven Glutathione Oxidation: Defining the Catalytic Role of Chalcopyrite Nanoparticles. J Phys Chem C Nanomater Interfaces. 2023;127(29):14146-14154.
[31] AN P, FAN F, GU D, et al. Photothermal-Reinforced and Glutathione-Triggered In Situ Cascaded Nanocatalytic Therapy. J Control Release. 2020;321:734-743.
[32] ZHAO J, TIAN Z, ZHAO S, et al. Insights into the Effect of Catalytic Intratumoral Lactate Depletion on Metabolic Reprogramming and Immune Activation for Antitumoral Activity. Adv Sci. 2023;10(4): 2204808.
[33] XIE H, LIU X, HUANG Z, et al. Nanoscale Zeolitic Imidazolate Framework (ZIF)-8 in Cancer Theranostics: Current Challenges and Prospects. Cancers (Basel). 2022;14(16):3935.
[34] COLUCCIA M, PARISSE V, GUGLIELMI P, et al. Metal-Organic frameworks (MOFs) as Biomolecules Drug Delivery Systems for Anticancer Purposes. Eur J Med Chem. 2022;244:114801.
[35] CHEN Y, LYU R, WANG J, et al. Metal-Organic Frameworks Nucleated by Silk Fibroin and Modified with Tumor-Targeting Peptides for Targeted Multimodal Cancer Therapy. Adv Sci. 2023;10(28):2302700.
[36] LEI C, LIU XR, CHEN QB, et al. Hyaluronic Acid and Albumin Based Nanoparticles for Drug Delivery. J Control Release. 2022;331:416-433.
[37] HASSN MESRATI M, SYAFRUDDIN SE, MOHTAR MA, et al. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules. 2021; 11(12):1850.
[38] ZHANG R, ZHAO X, JIA A, et al. Hyaluronic Acid-Based Prodrug Nanomedicines for Enhanced Tumor Targeting and Therapy: A Review. Int J Biol Macromol. 2023;249:125993.
|