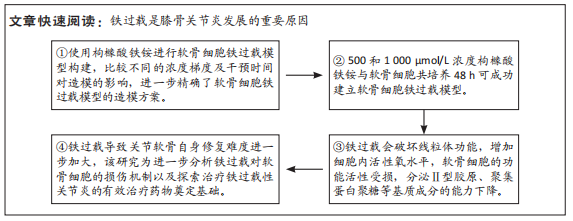
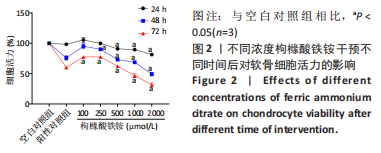
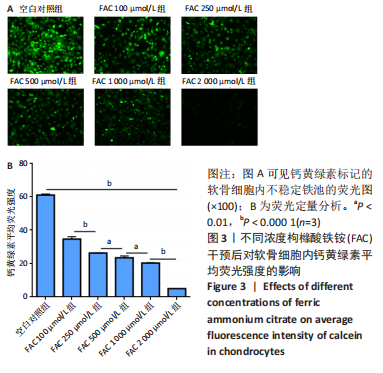
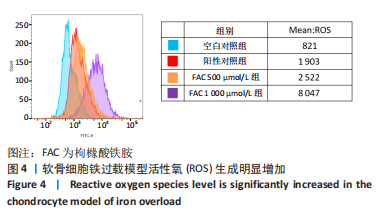
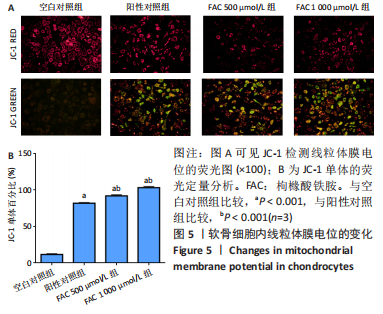
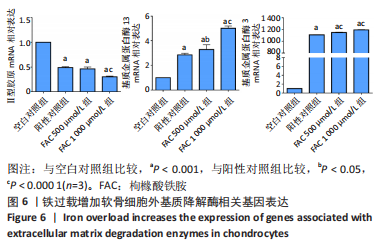
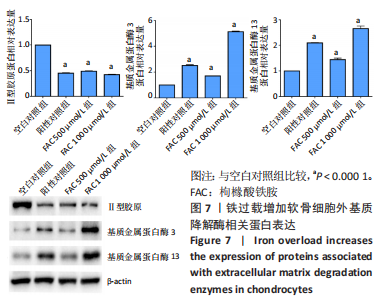
[1] BIJLSMA JW, BERENBAUM F, LAFEBER FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115-2126.
[2] HUNTER DJ, BIERMA-ZEINSTRA S. Osteoarthritis. Lancet. 2019;393(10182):1745-1759.
[3] WENHAM CY, CONAGHAN PG. New horizons in osteoarthritis. Age Ageing. 2013; 42(3):272-278.
[4] LEE AS, ELLMAN MB, YAN D, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440-447.
[5] 张荣,张向东,赵明宇.膝骨关节炎发病机制及治疗进展[J].风湿病与关节炎,2019,8(5):68-72.
[6] 中华中医药学会骨伤科分会膝痹病(膝骨关节炎)临床诊疗指南制定工作组.中医骨伤科临床诊疗指南·膝痹病(膝骨关节炎)[J].康复学报,2019, 29(3):1-7.
[7] GLYN-JONES S, PALMER AJ, AGRICOLA R, et al. Osteoarthritis. Lancet. 2015;386 (9991):376-387.
[8] GOLDRING MB, OTERO M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471-478.
[9] GOLDRING MB, FUKUO K, BIRKHEAD JR, et al. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994;54(1):85-99.
[10] JING X, DU T, LI T, et al. The detrimental effect of iron on OA chondrocytes: Importance of pro-inflammatory cytokines induced iron influx and oxidative stress. J Cell Mol Med. 2021;25(12):5671-5680.
[11] ROOSENDAAL G, TEKOPPELE JM, VIANEN ME, et al. Articular cartilage is more susceptible to blood induced damage at young than at old age. J Rheumatol. 2000;27(7):1740-1744.
[12] CARROLL GJ, SHARMA G, UPADHYAY A, et al. Ferritin concentrations in synovial fluid are higher in osteoarthritis patients with HFE gene mutations (C282Y or H63D). Scand J Rheumatol. 2010;39(5):413-420.
[13] CAMACHO A, SIMÃO M, EA HK, et al. Iron overload in a murine model of hereditary hemochromatosis is associated with accelerated progression of osteoarthritis under mechanical stress. Osteoarthritis Cartilage. 2016;24(3):494-502.
[14] HOOIVELD MJ, ROOSENDAAL G, VIANEN ME, et al. Immature articular cartilage is more susceptible to blood-induced damage than mature articular cartilage: an in vivo animal study. Arthritis Rheum. 2003;48(2):396-403.
[15] KENNISH L, ATTUR M, OH C, et al. Age-dependent ferritin elevations and HFE C282Y mutation as risk factors for symptomatic knee osteoarthritis in males: a longitudinal cohort study. BMC Musculoskelet Disord. 2014;15:8.
[16] CARROLL GJ. Primary osteoarthritis in the ankle joint is associated with finger metacarpophalangeal osteoarthritis and the H63D mutation in the HFE gene: evidence for a hemochromatosis-like polyarticular osteoarthritis phenotype. J Clin Rheumatol. 2006;12(3):109-113.
[17] 郭州,彭雅文,孙凯,等.铁代谢及其与骨关节炎关系的研究进展[J].骨科, 2020,11(5):457-462.
[18] MUSUMECI G, CASTROGIOVANNI P, TROVATO FM, et al. Biomarkers of Chondrocyte Apoptosis and Autophagy in Osteoarthritis. Int J Mol Sci. 2015;16(9): 20560-20575.
[19] THOMAS RS, CLARKE AR, DUANCE VC, et al. Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis. Arthritis Res Ther. 2011;13(6):R203.
[20] RAHMATI M, NALESSO G, MOBASHERI A, et al. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res Rev. 2017;40:20-30.
[21] SHEN S, WU Y, CHEN J, et al. CircSERPINE2 protects against osteoarthritis by targeting miR-1271 and ETS-related gene. Ann Rheum Dis. 2019;78(6):826-836.
[22] TAKÁCS-BUIA L, IORDACHEL C, EFIMOV N, et al. Pathogenesis of osteoarthritis: chondrocyte replicative senescence or apoptosis? Cytometry B Clin Cytom. 2008;74(6):356-362.
[23] ANDREWS NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986-1995.
[24] ONG-AJYOOTH L, MALASIT P, ONG-AJYOOTH S, et al. Renal function in adult beta-thalassemia/Hb E disease. Nephron. 1998;78(2):156-161.
[25] CRICHTON RR, WARD RJ. Iron species in iron homeostasis and toxicity. Analyst. 1995;120(3):693-697.
[26] SUANTAWEE T, TANTAVISUT S, ADISAKWATTANA S, et al. Oxidative stress, vitamin e, and antioxidant capacity in knee osteoarthritis. J Clin Diagn Res. 2013;7(9):1855-1859.
[27] ZHANG W, SUN G, AITKEN D, et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology (Oxford). 2016;55(9):1566-1574.
[28] LOESER RF, COLLINS JA, DIEKMAN BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412-420.
[29] XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369-379.
[30] DE VALK B, MARX JJ. Iron, atherosclerosis, and ischemic heart disease. Arch Intern Med. 1999;159(14):1542-1548.
[31] TOYOKUNI S, OKADA S, HAMAZAKI S, et al. Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitrilotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res. 1990;50(17):5574-5580.
[32] BRESGEN N, ECKL PM. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules. 2015;5(2):808-847.
[33] BLANCO FJ, REGO I, RUIZ-ROMERO C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7(3):161-169.
[34] VAN VULPEN LF, SCHUTGENS RE, COELEVELD K, et al. IL-1β, in contrast to TNFα, is pivotal in blood-induced cartilage damage and is a potential target for therapy. Blood. 2015;126(19):2239-2246.
[35] ZHANG DL, GHOSH MC, ROUAULT TA. The physiological functions of iron regulatory proteins in iron homeostasis - an update. Front Pharmacol. 2014;5:124.
[36] WANG T, HE C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50.
[37] BOLDUC JA, COLLINS JA, LOESER RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73-82.
[38] KUANG F, LIU J, TANG D, et al. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front Cell Dev Biol. 2020;8:586578.
|