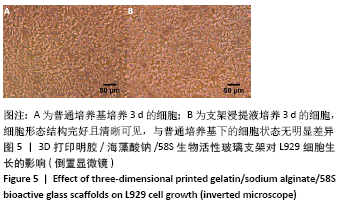
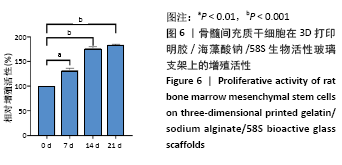
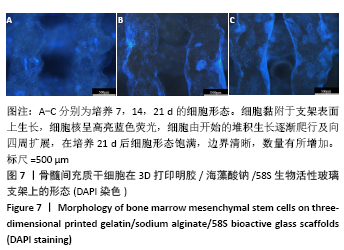
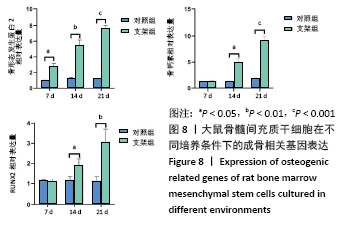
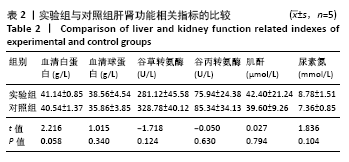
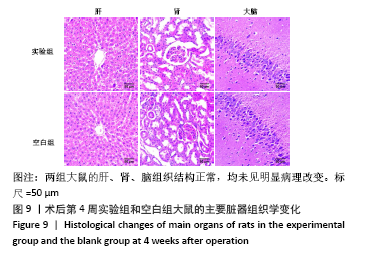
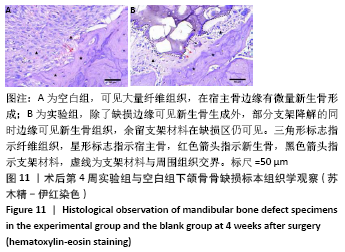
[1] HWANG KS, CHOI JW, KIM JH, et al. Comparative Efficacies of Collagen-Based 3D Printed PCL/ PLGA/β-TCP Composite Block Bone Grafts and Biphasic Calcium Phosphate Bone Substitute for Bone Regeneration. Materials (Basel). 2017;10(4):421.
[2] MANO T, AKITA K, FUKUDA N, et al. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J Biomed Mater Res B Appl Biomater. 2020;108(4):1450-1459.
[3] LEE Y, CHAN Y, HSIEH S, et al. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int J Mol Sci. 2019; 20(20):5015.
[4] ZHU T, CUI Y, ZHANG M, et al. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact Mater. 2020;5:584-601.
[5] WANG C, HUANG W, ZHOU Y, et al. 3D printing of bone tissue engineering scaffolds.Bioact Mater. 2020;5: 82-91.
[6] 刘洋,王欢,朱晔,等.骨组织工程支架材料研究进展[J].临床口腔医学杂志,2019,35(10):637-639.
[7] 范海霞,王宏,程焕芝,等.3D打印HAP-GEL支架复合BMSCs和HUVECs细胞修复兔颅骨缺损[J].临床口腔医学杂志,2020,36(4):199-202.
[8] ASHAMMAKHI N, HASAN A, KAARELA O, et al. Advancing Frontiers in Bone Bioprinting. Adv Healthc Mater. 2019;8:e1801048.
[9] ZHANG L, YANG G, JOHNSON B, et al. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16-33.
[10] SOORIYAARACHCHI D, MINIÈRE H, MAHARUBIN S, et al. Hybrid Additive Microfabrication Scaffold Incorporated with Highly Aligned Nanofibers for Musculoskeletal Tissues. Tissue Eng Regen Med. 2019;16:29-38.
[11] GU Q, HAO J, LU Y, et al. Three-dimensional bio-printing. Sci China Life Sci. 2015;58:411-419.
[12] PUROHIT S, SINGH H, BHASKAR R, et al. Gelatin-alginate-cerium oxide nanocomposite scaffold for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;116:111111
[13] ZHENG A, CAO L, LIU Y, et al. Biocompatible silk/calcium silicate/sodium alginate composite scaffolds for bone tissue engineering. Carbohydr Polym. 2018;199:244-255.
[14] YU H, ZHANG X, SONG W, et al. Effects of 3-dimensional Bioprinting Alginate/ Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells. J Endod. 2019;45:706-715.
[15] YE Q, ZHANG Y, DAI K, et al. Three dimensional printed bioglass/gelatin/alginate composite scaffolds with promoted mechanical strength, biomineralization, cell responses and osteogenesis. J Mater Sci Mater Med. 2020;31(9):77.
[16] 吕孝帅,李正茂,杨雪超.生物活性玻璃58 S/丝素蛋白膜促进人牙髓干细胞增殖与分化[J].牙体牙髓牙周病学杂志,2015(10):577-583.
[17] YAO Q, LIU H, LIN X, et al. 3D Interpenetrated Graphene Foam/58S Bioactive Glass Scaffolds for Electrical-Stimulation-Assisted Differentiation of Rabbit Mesenchymal Stem Cells to Enhance Bone Regeneration. J Biomed Nanotechnol. 2019;15:602-611.
[18] DING Y, LI W, MÜLLER T, et al. Electrospun Polyhydroxybutyrate/Poly(ε-caprolactone)/58S Sol-Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2016;8:17098-17108.
[19] WU J, MIAO G, ZHENG Z, et al. 3D printing mesoporous bioactive glass/sodium alginate/gelatin sustained release scaffolds for bone repair. J Biomater Appl. 2019;33:755-765.
[20] YANG S, XU S, ZHOU P, et al. Siliceous mesostructured cellular foams/poly (3-hydroxybutyrate–co-3 -hydroxyhexanoate) composite biomaterials for bone regeneration. Int J Nanomedicine. 2014;9:4795-4807.
[21] KIM J, PARK J, MUTHUKUMAR T, et al. Accelerating bone defects healing in calvarial defect model using 3D cultured bone marrow-derived mesenchymal stem cells on demineralized bone particle scaffold. J Tissue Eng Regen Med. 2020;14:563-574.
[22] 潘朝晖,栾兆新,高朋.两种交联剂对β-磷酸三钙明胶复合骨支架理化及生物学性能影响的比较[J].中国组织工程研究,2018,22(6):833-839.
[23] KIKUCHI M, MATSUMOTO H, YAMADA T, et al. Glutaraldehyde cross-linked hydroxyapatite/collagen self-organized nanocomposites. Biomaterials. 2004;25:63-69.
[24] PENG Y, GLATTAUER V, RAMSHAW J. Stabilisation of Collagen Sponges by Glutaraldehyde Vapour Crosslinking.Int J Biomater. 2017;2017:8947823.
[25] SUESCA E, DIAS A, BRAGA M, et al. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater Sci Eng C Mater Biol Appl. 2017;77:333-341.
[26] SUN X, MA C, GONG W, et al. Biological properties of sulfanilamide-loaded alginate hydrogel fibers based on ionic and chemical crosslinking for wound dressings.Int J Biol Macromol. 2020;157:522-529.
[27] GHADERI G, SAHEBGHADAM L, KORDI T, et al. The enhancement of differentiating adipose derived mesenchymal stem cells toward hepatocyte like cells using gelatin cryogel scaffold. Biochem Biophys Res Commun. 2017;491:1000-1006.
[28] SIVASHANKARI P, MOORTHI A, ABUDHAHIR K, et al. Preparation and characterization of three-dimensional scaffolds based on hydroxypropyl chitosan-graft-graphene oxide. Int J Biol Macromol. 2018;110:522-530.
[29] 王闯建,刘宏建,张春霖,等.喷墨全包芯骨支架材料的制备及生物安全性研究[J].中华实验外科杂志,2019,36(5):915-918
[30] CHEN Y, KAWAZOE N, CHEN G. Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 2018;67:341-353.
[31] HOPPE A, GÜLDAL N, BOCCACCINI A. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757-2774.
[32] GERHARDT L, BOCCACCINI A. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials (Basel). 2010;3(7):3867-3910. |