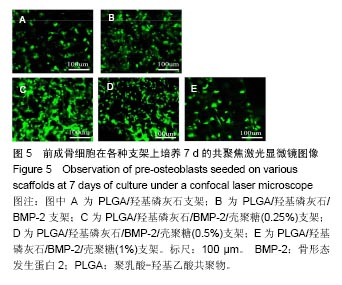
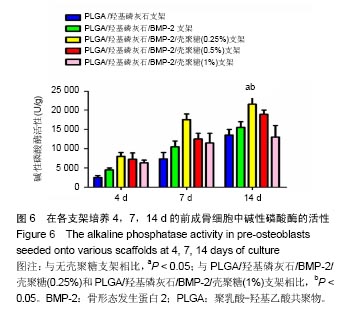
| [1]Reichert JC, Epari DR, Wullschleger ME, et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng Part B Rev. 2010;16(1):93-104.[2]Wang C, Wang Z, Li A, et al. Repair of segmental bone-defect of goat's tibia using a dynamic perfusion culture tissue engineering bone. J Biomed Mater Res A. 2010;92(3): 1145-1153.[3]李建军,赵群,王欢,等.基因修饰的组织工程骨联合带血管蒂骨膜移植修复长段骨缺损的研究[J].中华整形外科杂志, 2007,23(6): 502-506.[4]Ami R. Amini, Cato T. et al. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng.2012; 40(5): 363–408.[5]Delabarde C, Plummer CJ, Bourban PE, et al. Biodegradable polylactide/hydroxyapatite nanocomposite foam scaffolds for bone tissue engineering applications. J Mater Sci Mater Med. 2012;23(6):1371-1385.[6]Tripathi A, Saravanan S, Pattnaik S, et al. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/ nano-copper-zinc for bone tissue engineering. Int J Biol Macromol. 2012;50(1):294-299.[7]Brun F, Turco G, Accardo A, et al. Automated quantitative characterization of alginate/hydroxyapatite bone tissue engineering scaffolds by means of micro-CT image analysis. J Mater Sci Mater Med. 2011;22(12):2617-2629.[8]Yu NY, Schindeler A, Little DG, et al. Biodegradable poly(alpha-hydroxy acid) polymer scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2010; 93(1):285-295.[9]Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13(5):927-938.[10]Jansen EJ, Sladek RE, Bahar H, et al. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomaterials. 2005;26(21):4423-4431.[11]Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749-4757.[12]Xiong JY, Li YC, Wang XJ, et al. Titanium-nickel shape memory alloy foams for bone tissue engineering. J Mech Behav Biomed Mater. 2008;1(3):269-273.[13]Warnke PH, Douglas T, Wollny P, et al. Rapid prototyping: porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng Part C Methods. 2009;15(2):115-124.[14]Ding F, Wu J, Yang Y, et al. Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A. 2010;16(12):3779-3790.[15]郝增涛,冯卫,郝廷,等.BMSCs来源成骨细胞和内皮细胞复合壳聚糖-羟基磷灰石多孔支架构建血管化组织工程骨研究[J].中国修复重建外科杂志,2012,26(4):489-494.[16]王新,刘玲蓉,张其清.纳米羟基磷灰石-壳聚糖骨组织工程支架的研究[J].中国修复重建外科杂志,2007,21(2):120-124.[17]Kim HW, Noh YJ, Koh YH, et al. Enhanced performance of fluorine substituted hydroxyapatite composites for hard tissue engineering. J Mater Sci Mater Med. 2003;14(10):899-904.[18]Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res.1999;44(4):446-455.[19]Link DP, van den Dolder J, Jurgens WJ, et al. Mechanical evaluation of implanted calcium phosphate cement incorporated with PLGA microparticles. Biomaterials. 2006; 27(28):4941-4947.[20]Zhang Q, Mochalin VN, Neitzel I, et al. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials. 2012; 33(20):5067-5075.[21]Sultana N, Wang M. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: surface modification and in vitro biological evaluation. Biofabrication. 2012;4(1):015003.[22]Ngiam M, Liao S, Patil AJ, et al. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone. 2009;45(1):4-16.[23]Hou J, Wang J, Cao L, et al. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Biomed Mater. 2012; 7(3):035002.[24]Oliveira JM, Rodrigues MT, Silva SS, et al. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials. 2006;27(36):6123-6137.[25]Park KH, Kim H, Moon S, et al. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108(6):530-537.[26]Sohier J, Daculsi G, Sourice S, et al. Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. J Biomed Mater Res A. 2010;92(3): 1105-1114.[27]Kempen DH, Lu L, Hefferan TE, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29(22):3245-3252.[28]袁泉.载骨形态发生蛋白2活性肽的纳米仿生骨基质材料的实验研究[D].华中科技大学,2017.[29]Waghela BN, Sharma A, Dhumale S, et al. Curcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cells. PLoS One.2015;10(2):e0117526.[30]刘莉,常红,黄国伟,等.体外培养大鼠成骨细胞实验模型的建立[J].天津医科大学学报,2004,10(1):39-42[31]许瑾,吴晶晶,王晓冬,王秀梅,韩倩倩.羟基磷灰石复合骨组织工程支架的研究进展[J].生物骨科材料与临床研究, 2016,13(2): 63-66.[32]Vinn SR, Hu Y, Sfeir C, et al. Gene therapy approaches for modulating bone regeneration. AdVDtug DeliV Rev. 2000;42: 12l-138.[33]Wang EA, Rosen V, Cordes P, et al. Purification and characterization of other distinct bone-inducing factors. Proc Nat Acad Sci USA. 2015;85:9484-9488. |
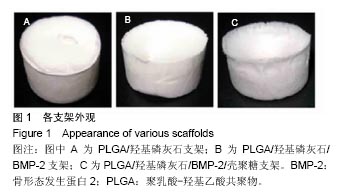
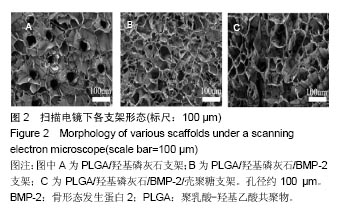
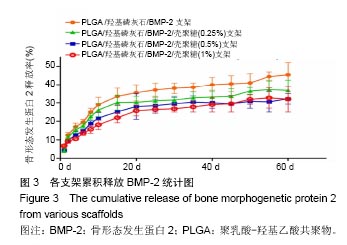
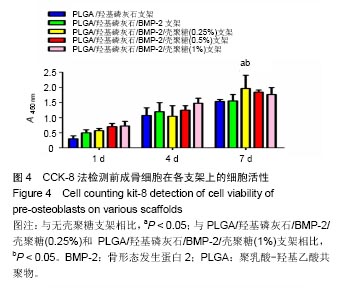
.jpg)