中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (35): 5676-5681.doi: 10.3969/j.issn.2095-4344.1990
• 组织构建临床实践 clinical practice in tissue construction • 上一篇 下一篇
吸盘型封闭式负压引流护创系统临床有效性和安全性评价:一项前瞻性、随机、开放、平行对照、非劣效性临床试验
袁亚翠1,刘琳娜2,刘喜文3,李跃军1,赵聪颖1,韩 琳1
- (空军军医大学唐都医院,1整形烧伤科,2药学部,陕西省西安市 710038; 3空军军医大学护理学院,陕西省西安市 710032)
Evaluation of the effectiveness and safety of closed negative pressure drainage nursing system (PU suction cup type): a perspective, randomized, open, parallel controlled and non-inferiority trial
Yuan Yacui1, Liu Linna2, Liu Xiwen3, Li Yuejun1, Zhao Congying1, Han Lin1
- (1Department of Plastic Surgery and Burn, 2Department of Pharmacy, Tangdu Hospital of Air Force Medical University, Xi’an 710038, Shaanxi Province, China; 3School of Nursing, Air Force Medical University, Xi’an 710032, Shaanxi Province, China)
摘要:
文章快速阅读:
.jpg) 文题释义:
封闭式负压引流护创材料套装(PU吸盘型)的构成:主要由PU型海绵、医用透明贴膜、吸盘型引流管、冲洗管、冲洗连接管、两通接头、吸引连接管、止流夹组成。
PU型海绵:主要成分为聚氨基甲酸酯简称聚氨酯,其孔径疏松,柔软而富有弹性,可使伤口部位的负压均匀分布。
文题释义:
封闭式负压引流护创材料套装(PU吸盘型)的构成:主要由PU型海绵、医用透明贴膜、吸盘型引流管、冲洗管、冲洗连接管、两通接头、吸引连接管、止流夹组成。
PU型海绵:主要成分为聚氨基甲酸酯简称聚氨酯,其孔径疏松,柔软而富有弹性,可使伤口部位的负压均匀分布。
.jpg) 文题释义:
封闭式负压引流护创材料套装(PU吸盘型)的构成:主要由PU型海绵、医用透明贴膜、吸盘型引流管、冲洗管、冲洗连接管、两通接头、吸引连接管、止流夹组成。
PU型海绵:主要成分为聚氨基甲酸酯简称聚氨酯,其孔径疏松,柔软而富有弹性,可使伤口部位的负压均匀分布。
文题释义:
封闭式负压引流护创材料套装(PU吸盘型)的构成:主要由PU型海绵、医用透明贴膜、吸盘型引流管、冲洗管、冲洗连接管、两通接头、吸引连接管、止流夹组成。
PU型海绵:主要成分为聚氨基甲酸酯简称聚氨酯,其孔径疏松,柔软而富有弹性,可使伤口部位的负压均匀分布。摘要
背景:吸盘型封闭式负压引流护创系统是近几年在负压封闭引流技术基础上研发的、具有持续密闭负压引流功能的创面治疗装置。正确使用、保证有效引流的前提下,与传统换药清创相比,便于医护人员实时监控创面愈合情况,可显著减少大剂量抗菌药物的应用及换药次数,进而降低治疗费用,缩短住院日,减轻患者痛苦。
目的:评价试验产品吸盘型封闭式负压引流护创系统的临床有效性及安全性,为其临床推广及产品应用注册提供理论及数据支持。
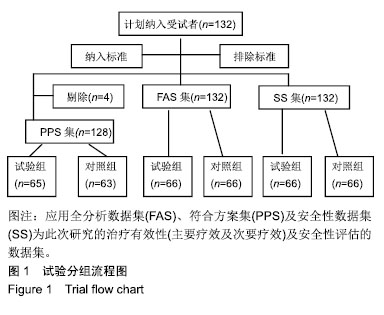
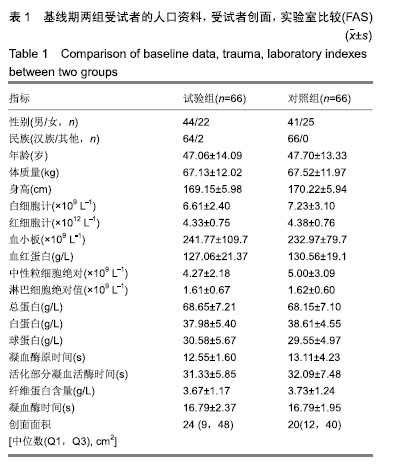
方法:将132例需负压封闭引流治疗的患者随机分为2组,试验组66例使用封闭式负压引流护创系统(PU)(型号:PU吸盘型),对照组66例使用封闭式负压引流套件(规格型号:Ⅱ-PU型)。评估治疗14 d的结局变化,主要结局指标:治疗14 d内治疗有效率;次要结局指标(首次使用,拆除或更换时):目测类比评分、操作满意度、产品物理性能;安全性评价指标:不良事件/反应发生率、并发症发生率及实验室检查指标。
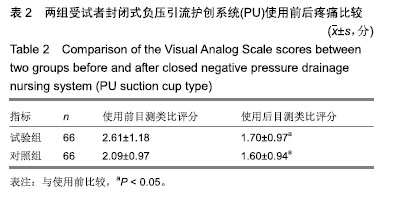
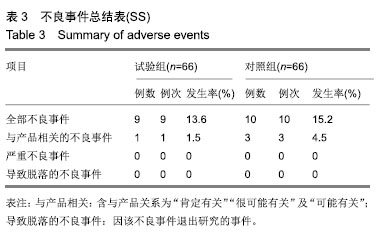
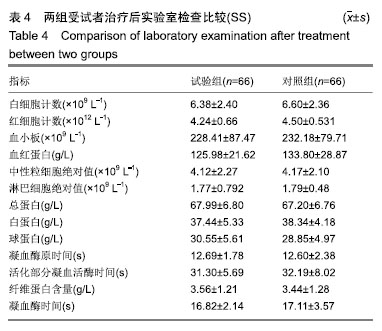
结果与结论:①有效性指标:治疗后14 d内两组有效率均为100%;与治疗前相比,治疗后(10±3) d两组目测类比评分均显著下降(P < 0.05),且治疗后两组间比较无显著性意义;②安全性指标:两组治疗前后实验室检测指标(血常规,肝功能,凝血4项)差异无显著性意义;两组产品操作满意度、产品物理性能均能100%满足临床使用;试验组和对照组产品均未出现并发症;整个试验期间,试验组66例患者中有9例(13.6%)发生不良事件,66例对照组受试者中有10例(15.2%)发生不良事件;③结果证实,封闭式负压引流护创材料套装(PU)用于创面引流的疗效与安全性与对照产品相当。试验于2016-09-13获得空军军医大学唐都医院医学伦理委员会批准,批准号:第201609-10号。
中图分类号:
.jpg) 文题释义:
封闭式负压引流护创材料套装(PU吸盘型)的构成:主要由PU型海绵、医用透明贴膜、吸盘型引流管、冲洗管、冲洗连接管、两通接头、吸引连接管、止流夹组成。
PU型海绵:主要成分为聚氨基甲酸酯简称聚氨酯,其孔径疏松,柔软而富有弹性,可使伤口部位的负压均匀分布。
文题释义:
封闭式负压引流护创材料套装(PU吸盘型)的构成:主要由PU型海绵、医用透明贴膜、吸盘型引流管、冲洗管、冲洗连接管、两通接头、吸引连接管、止流夹组成。
PU型海绵:主要成分为聚氨基甲酸酯简称聚氨酯,其孔径疏松,柔软而富有弹性,可使伤口部位的负压均匀分布。