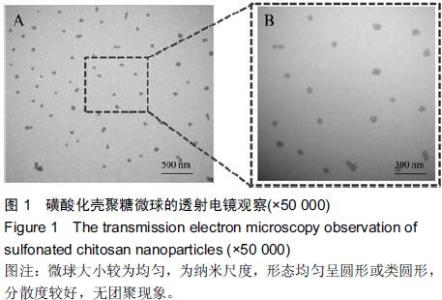
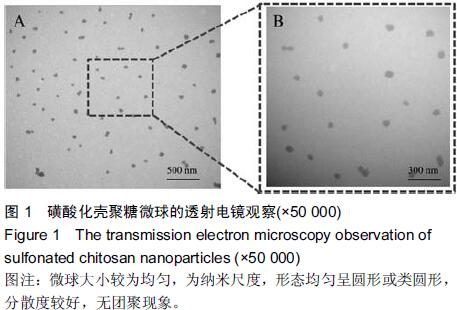
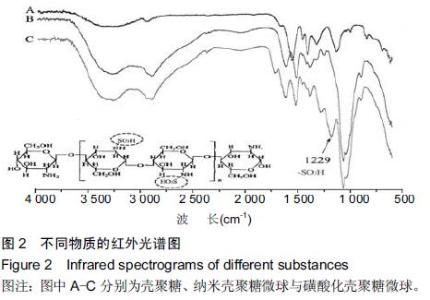
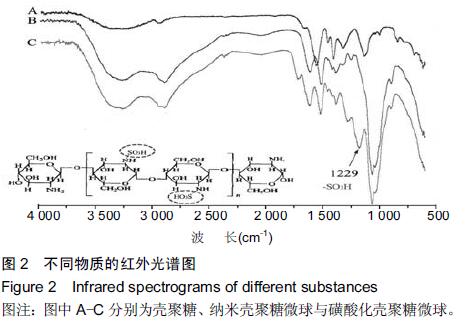
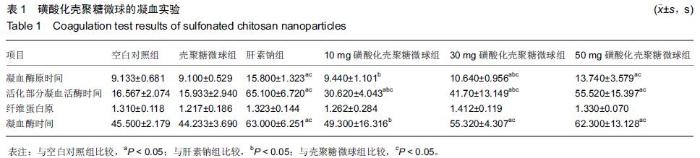
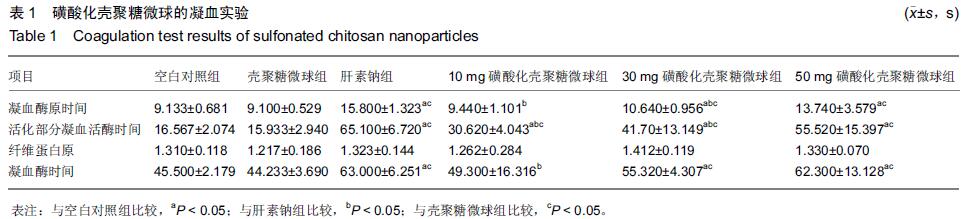
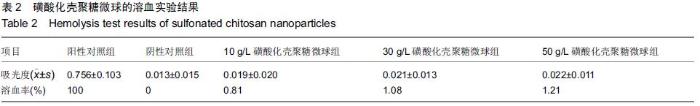
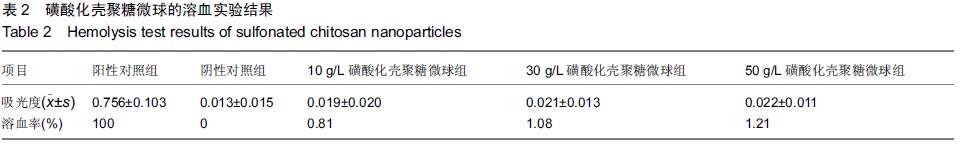
| [1] Bilati U,Allemann E,Doelker E.Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles.Eur J Pharm Biopharm.2005;59(3):375-388.
[2] Huang G,Gao J,Hu ZB,et al.Controlled drug release from hydrogel nanoparticle networks.J Control Release.2004; 94(2-3):303-311.
[3] Bonian P,Petnova T,Manolova N,et al.pH-senstive hydrogels composed of chitosan and polyaerylamide: enzymatice degradation.J Bioact Compat Polym.2004;19:197-208.
[4] Chang KL,Tai MC,Cheng EH.Kinetics and products of the degradation of chitosan by hydrogen peroxide.J Agric Food Chem.2001;49(10):4845-4851.
[5] Vanfrvord PJ,Matthw HW,Desilva SP,et al.Evaluation of the biocompatibility of a chitosan scaffold in mice.J Biomed Mater Res.2002;59(3):585-590.
[6] Rao SB,Shama CP.Use of chitosan as a biomaterial: studies on its safety and hemostatic potential.J Biomed Mater Res. 1997;34(1):21.
[7] Chou TC,Li CY,Lee AR,et al.Mechanism of inhibition of platelet aggregation by HCL-31D.Eur J Pharmacol.2000; 387(2):125-131.
[8] 尹刚,侯春林.浅析壳聚糖的止血作用及机理[J].生物骨科材料与临床研究,2009,6(4):18-23.
[9] Dodane V,Khan AM,Merwin JR.Efect of chitosan on epithelia permeability and structure.Int J Pharm.1999; 182(1):21-32.
[10] 王琰,李正荣,潘飞燕,等.纳米脂质体包裹胰岛素经Caco-2细胞转运的研究[J].中国药理学通报,2005,21(1):78-81.
[11] Fernandez UR,Calvo P,Remunan LC,et al.Enhancement of nasal absorption of insulin using chitosan nanoparticles.Pharm Res.1999;16(10):1576-1580.
[12] Mitra S,Caur U,Ghosh PC,et al.Tumor targeted delivery of encapsulated dextran -doxorubicin conjugate using chitosan nanoparticles as carrier.J Control Release. 2001;74(1-3): 317-323.
[13] 王斌.壳聚糖的硫酸/氯磺酸酯化[D].成都:西南石油大学,2004.
[14] 杨越冬.磺化6-羧基壳聚糖的制备、表征及血液相容性研究[D].天津:天津大学,2007.
[15] 蒋挺大.甲壳素[M].北京:化学工业出版社,2003:128.
[16] Amiji MM.Platelet adhesion and activation on an amphoteric chitosan derivative bearing sulfonate groups.Colloids Surf B Biointerfaces.1998;10(5):263-271.
[17] 罗华丽,臧剑甬,张秀娟.壳聚糖载药微球的制备和体外释放研究[J].河南科学,2011,29(1):20-24.
[18] 王世敏,许祖勋,傅晶.纳米材料制备技术[M].北京:化学工业出版社,2002:1-6.
[19] 许加超,肖英龙.壳聚糖硫酸醋在药物中的应用[J].海洋科学, 1996, 20(2):2-4.
[20] Baumann H,Faust V.Concepts for improved regioselective placement of O-sulfo, N-sulo, N-acetyl and N-carboxymethyl groups in chitosan derivatives.Carbohydrate Res. 2001; 331(1):43-44.
[21] 王声宇,张文清.壳聚糖硫酸酯的合成及其抑菌作用[J].功能高分子学报,2007,19(4): 440-442.
[22] 张虹.壳聚糖的改性及其抗凝血活性研究[D].安徽:安徽工程大学,2014. |