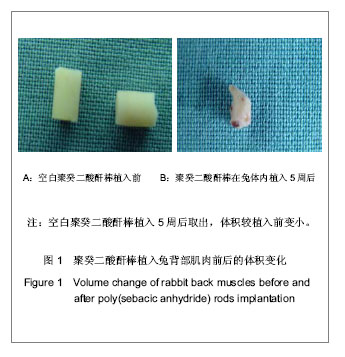
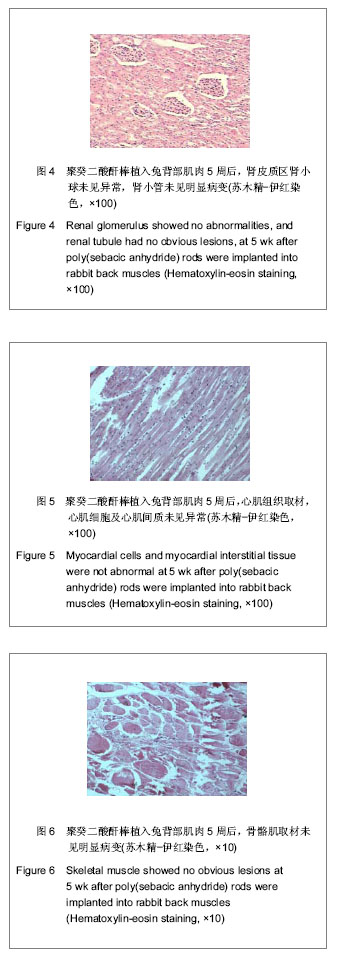
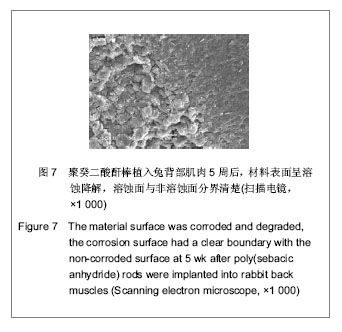
| [1] Zhou ZB,Huang KX,Chen ZX,et al.Beijing Shengwu Yixue Goncheng. 2001;20(1):76-79.周志彬,黄开勋,陈泽宪,等.生物可降解高分子材料—聚酸酐[J].北京生物医学工程,2001,20(1):76-79.[2] Yu YT,Zhang XD.Tianjin:Tianjin Daxue Chubanshe. 2000: 12-39.俞耀庭,张兴栋.生物医用材料[M].天津:天津大学出版社,2000: 12-39.[3] Liu YH,Wen SL,Shang ZW.Zhongguo Shengwu Yixue Gongcheng Xuebao. 2000;19(1):53-59.刘月辉,文三立,尚尊文.复合活性羟基磷灰石陶瓷的研制及其生物相容性研究[J].中国生物医学工程学报,2000,19(1):53-59.[4] Sun ML,Hu YY,Jia XB,et al.Disi Junyi Daxue Xuebao. 2001; 22(11):1006-1009.孙明林,胡蕴玉,贾新斌,等.磷酸钙骨水泥 /骨形态发生蛋白复合人工骨的生物相容性[J].第四军医大学学报,2001,22(11): 1006-1009.[5] Du Y,Guo HY.Guowai Yiyao:Kangshengsu Fence. 2001;22(1): 34-43.杜煜,郭惠云. 广谱、高效、低毒的新喹诺酮类抗菌药加替沙星[J].国外医药:抗生素分册,2001,22(1):34-43.[6] Liu JY,Tian ZM,Guo HY.Zhongguo Yiyao Gongye Zazhi. 2001; 32(10):433-437.刘九雨,田治明,郭惠元.加替沙星新合成法的探索[J].中国医药工业杂志,2001,32(10):433-437.[7] Zhou J,Jiang XH,Huang LF,et al.Zhongguo Kangshengsu Zazhi. 2004;29(3):146-148.周静,蒋学华,黄龙飞,等.加替沙星制剂中有关物质检查与含量测定的高效液相色谱法[J].中国抗生素杂志,2004,29(3):146-148.[8] Langer R. Biomaterials in drug delivery and tissue engineering: One laboratory's experience. Acc Chem Res. 2000;33(2):94-101.[9] Sampath P,Brem H. Implantable slow-release chemotherapeutic polymers for the treatment of malignant brain tumors. Cancer Control.1998;5(2):130-137.[10] Cheng ZM,Zhang J.Chongqing Yixue. 2006;35(3):258-259.程祝海,张君.加替沙星序贯治疗下呼吸道细菌感染56例疗效观察[J].重庆医学,2006,35(3):258-259.[11] Cai QX,Zhu KJ,Chen D,et al.Synthesis, characterization and in vitro release of 5-aminosalicylic acid and 5-acetyl aminosalicylic acid of polyanhydride P(CBFAS). Eur J Pharm Biopharm. 2003;55(2):203-208.[12] Berkland C,Kipper MJ,Narasimhan B,et al.Micro- sphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. J Controlled Release. 2004;94(1):129-141. |