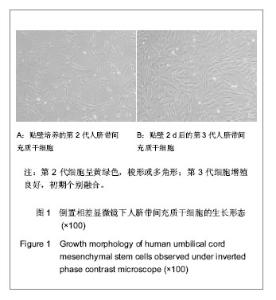
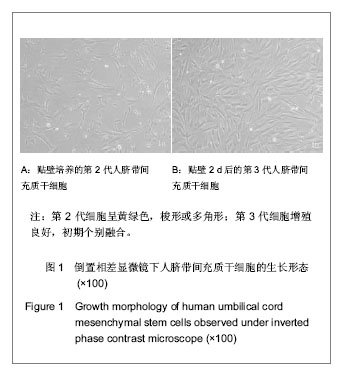
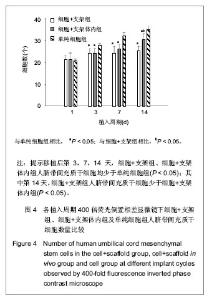
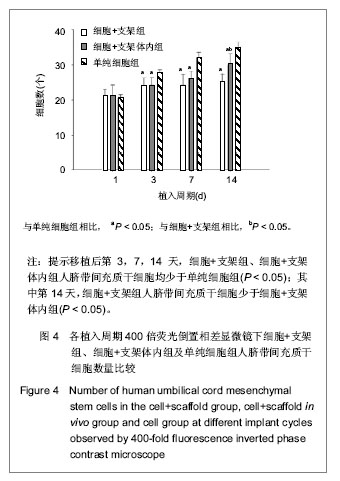
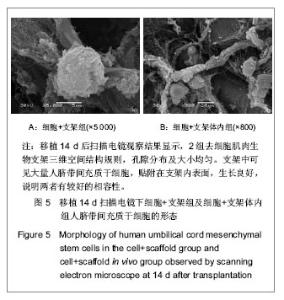
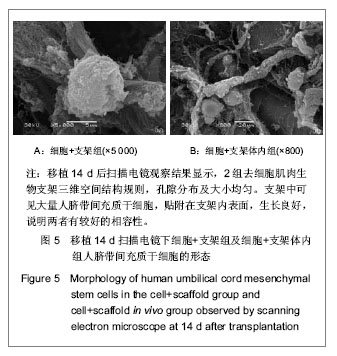
| [1] Heldner MR, Arnold M, Nedeltchev K, et al. Vascular Diseases of the Spinal Cord: A Review. Curr Treat Options Neurol. 2012;14(6):509-520. [2] Fehlings MG, Tighe A. Spinal Cord Injury: The Promise of Translational Research. Neurosurg Focus. 2008;25(5):1. [3] Li L, Lü G, Wang YF, et al. Glial cell-derived neurotrophic factor mRNA expression in a rat model of spinal cord injury following bone marrow stromal cell transplantation. Neural Regen Res 2008;3(10):1056-1059.[4] Han MY, Feng SQ, Li H, et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14(19):3483-3489.韩明远, 冯世庆, 李辉, 等. 移植人脐带间充质干细胞修复大鼠脊髓损伤[J]. 中国组织工程研究与临床康复, 2010,14(19): 3483-3489.[5] Zou J, Gan MF, Zhu XS, et al. Protective effects of human umbilical cord mesenchymal stem cell vein transplantation against spinal cord ischemia/reperfusion injury in rats. Neural Regener Res.2010;5(3):7.[6] Guo YW, Ke YQ, Li M, et al. Human Umbilical Cord-Derived Schwann-Like Cell Transplantation Combined with Neurotrophin-3 Administration in Dyskinesia of Rats with Spinal Cord Injury. Neurochem Res. 2011;36(5):783-792. [7] Han MY. Tianjin Yike Daxue. 2010.韩明远. 不同途径移植人脐带间充质干细胞修复大鼠脊髓损伤的研究[D]. 天津医科大学,2010.[8] Wang XH, Shen YX, Fan ZH, et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15(25): 4719-4722.王新宏, 沈忆新, 范志海, 等. 组织工程支架材料在脊髓损伤修复中的应用[J]. 中国组织工程研究与临床康复, 2011,15(25): 4719-4722.[9] Xue H, Chen D, Zhang XY, et al. Xi′an Jiaotong Daxue Xuebao. 2009;30(2):5.薛辉, 陈东, 张秀英, 等. 化学去细胞肌肉促进大鼠脊髓半横断后的轴突再生[J]. 西安交通大学学报,2009,30(2):5.[10] The Ministry of Science and Technology of the People's Republic of china.Guidance Suggestions for the Care and Use of Laboratory Animals. 2009.[11] Xiao HT, Niu XH, Liu Y. Zhengzhou Daxue Xuebao. 2010; 45(2):4.肖宏涛, 牛希华, 刘毅. 人脐带间充质干细胞的体外分离、培养及诱导分化[J]. 郑州大学学报,2010,45(2):4.[12] Liu Y, Xiao HT. Zhonghua Yixue Meixue Meirong Zazhi. 2010; 16(1):4.刘毅,肖宏涛.人脐带间充质干细胞与蚕丝素多孔支架的体外复合培养[J]. 中华医学美学美容杂志,2010,16(1):4.[13] Mligiliche N, Kitada M, Ide C. Grafting of detergent-denatured skeletal muscles provides effective conduits for extension of regenerating axons in the rat sciatic nerve. Arch Histol Cytol. 2001;64(1):29-36. [14] Arai T, Kanje M, Lundborg G, et al. Axonal outgrowth in muscle grafts made acellular by chemical extraction. Restor Neurol Neurosci. 2000;17(4):165-174. [15] Wei XK, Wen YM, Li H, et al. Zhongguo Jiaoxing Waike Zazhi. 2012;(20):1874-1877.魏祥科, 文益民, 李含, 等. 骨髓间充质干细胞与去细胞肌肉生物支架体外相容性研究[J]. 中国矫形外科杂志, 2012, (20): 1874-1877.[16] Zhang XY, Xue H, Liu JM, et al. Chemically extracted acellular muscle: A new potential scaffold for spinal cord injury repair. J Biomed Mater Res A. 2012;100A(3):578-587. [17] Li PJ, Xu ST. Zhongguo Jizhu Jisui Zazhi. 2000;10(4):5.李培建, 胥少汀. 肌基膜管移植及神经生长因子对脊髓横断性损伤的修复作用[J]. 中国脊柱脊髓杂志,2000,10(4):5.[18] Guo SZ, Ren XJ, Wu B, et al. Preparation of the acellular scaffold of the spinal cord and the study of biocompatibility. Spinal Cord. 2010;48(7):576-581. [19] Yin WH, Lu KW, Jin DD. Morphology of spinal cord extracellular matrix-derived acellular scaffolds fabricated in rats. Neural Regen Res. 2011;6(10):767-771.[20] Fehlings MG, Vawda R. Cellular Treatments for Spinal Cord Injury: The Time is Right for Clinical Trials. Neurotherapeutics. 2011;8(4):704-720. [21] Bajada S, Mazakova I, Richardson JB, et al. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2(4):169-183. [22] Wong RSY. Mesenchymal Stem Cells: Angels or Demons? J Biomed Biotechnol. 2011;2011:459510. [23] Hilfiker A, Kasper C, Hass R, et al. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg.2011;396(4):489-497. [24] Ma XH, Feng K, Shi BY. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15(32):6064-6067.马锡慧, 冯凯, 石炳毅. 人脐带间充质干细胞生物学特性及其研究进展[J]. 中国组织工程研究与临床康复,2011,15(32): 6064-6067.[25] Moroni L, Fornasari PM. Human mesenchymal stem cells: A bank perspective on the isolation, characterization and potential of alternative sources for the regeneration of musculoskeletal tissues. J Cell Physiol. 2013;228(4):680-687. [26] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [27] Yang CC, Shih YH, Ko MH, et al. Transplantation of Human Umbilical Mesenchymal Stem Cells from Wharton's Jelly after Complete Transection of the Rat Spinal Cord. Plos One. 2008;3(10):e3336. |