Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (12): 2241-2248.doi: 10.3969/j.issn.2095-4344.2013.12.022
Previous Articles Next Articles
Performance and evaluation of lung sustained-release microspheres for the treatment of lung diseases
Wang Shi-shou1, Wu Gang2
- 1 Shengli Oilfield Central Hospital, Dongying 257000, Shandong Province, China
2 Hekou District Drilling Hospital, Dongying 257000, Shandong Province, China
-
Received:2012-12-18Revised:2013-02-10Online:2013-03-19Published:2013-03-19 -
About author:Wang Shi-shou★, Master, Associate chief physician, Shengli Oilfield Central Hospital, Dongying 257000, Shandong Province, China shishouw@163.com
CLC Number:
Cite this article
Wang Shi-shou1, Wu Gang. Performance and evaluation of lung sustained-release microspheres for the treatment of lung diseases[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(12): 2241-2248.
share this article

1 药物控制释放系统的发展阶段及优势 近年来在医用高分子领域内,高分子药物控制释放体系的研究和开发迅速开展,药物控制释放系统就是将药理活性分子与天然或合成高分子载体结合或复合,使用后再不降低原来药效及抑制不良反应的情况下,以适当的浓度和持续时间,导向聚集到患病的器官、细胞部位以充分发挥原来的药物疗效的体系。它是由药物和药物载体组成,通过改变载体材料的类型、组成方式等条件,来调节药物在载体材料中的扩散速度和载体材料的降解溶蚀行为,最终获得理想的控制释放效果。由于药物是在靶部位释放,可以提高靶组织的药理作用强度和降低全身的不良反应。目前,对多种靶向给药系统的靶向机制、制备方法、特性、体内分布和代谢规律等都有了较为清楚的认识。但是,在生产和临床上的应用还存在不少问题,如载药量小的问题,稳定性差的问题,临床给药的制剂学问题,体内代谢动力学模型问题,体内生理作用问题等,这些都是缓释微球等胶体型靶向给药系统需要研究解决的问题。因此,药物控制释放系统是在传统制剂的基础上,随着材料学的发展以及新的药物检测方法的出现而出现的,相关领域的学者通过把药物剂型划分为4个阶段,以体现药物控制释放系统的历史沿革。"


2 制备微球载体材料及特点 理想的微球应是大小均匀的球形微粒,互不粘连,分散性好,便于制成各种制剂[8-9]。微球的结构随制备方法不同而有差异,通常凝聚法与辐射聚合法所制得的微球为球形镶嵌型,乳化分散法与聚合法所制得的微球是球形膜壳型。微球的粒径大小直接影响到微球中药物的释放及生物利用度、微球的载药量以及体内分布的靶向性[10]。例如随着微球粒径的增大,载药量随之增大,但药物从微球中的释放速度减慢。微球载体材料应具备的条件:①应具有良好的生理相容性,不引起血象的任何变化,不产生过敏反应。②靶向微球载体材料应能增加药物的定向性和在靶区的滞留性,以及对组织和细胞膜的渗透性和对癌组织的亲和性,以提高药物在靶区的有效浓度,维持较长的有效时间,增强抗癌作用。③载体进入靶区后,能按要求释放药物,释药后又具有良好的生物降解性,即可经体内代谢,变成无毒物质排出体外。④与药物有足够的结合或亲和能力以及具有较大的载药能力,能增加药物的稳定性,降低其毒副作用。根据微球制备所用高分子载体材料不同,微球又可分为天然高分子微球和合成聚合物微球。常用的微球载体材料[11]有如下7种: 淀粉:淀粉无毒、无抗原性,在体内可由淀粉酶降解,在生物体内具有一定的可变形性,其骨架在降解崩溃前的载药能力可保持较长时间,因其不溶于水,故淀粉微球常用作动脉栓塞微球来暂时阻塞小动脉血管。 壳聚糖:是一种聚阳离子多糖,因具有良好的物理化学特性、生物相容性和生物可降解性,来源丰富,毒副作用小,因此,用壳聚糖制备的具有负载、靶向、控释等作用的壳聚糖微球作为药物载体则明显优于一般材料。 明胶:明胶作为微球载体材料,无不良反应,无免疫原性,具有生物可降解性,在体内可生物降解,是目前常用微球载体材料之一,可口服和注射。 白蛋白[12]:从人或动物血液中分离提取而得。白蛋白是体内的生物降解物质,注入肌体后,在肌体的作用下逐渐降解后清除,性能稳定,无毒、无抗原性,是一种较理想的微球载体材料。 聚乳酸:常用作缓释骨架材料、微囊囊膜材料和微球载体材料,无毒、安全,在体内可慢慢降解为乳酸,最后成为水和二氧化碳。 聚酰胺:由二元酸与二胺类,或由氨基酸在催化剂的作用下聚合而制得的结晶形颗粒。对大多数化学物质稳定,无毒、安全,在体内不分解,不吸收,常供动脉栓塞给药或口服给药。 聚丙烯:由丙烯单体溶于有机溶剂,在引发剂作用下聚合而成的乳白色轻质颗粒,化学性质稳定,不溶于水,能溶于三氯乙烷、热的萘烷等有机溶剂,除用作包装材料外,可用作磁性微球载体材料、微囊囊膜材料及缓释骨架材料等。"

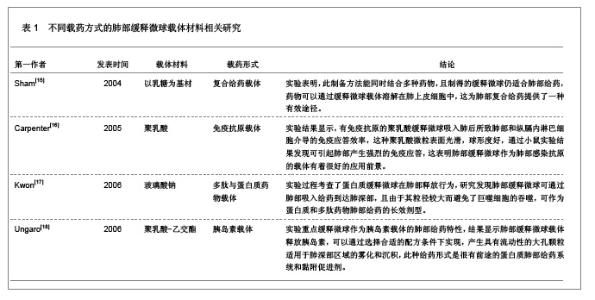
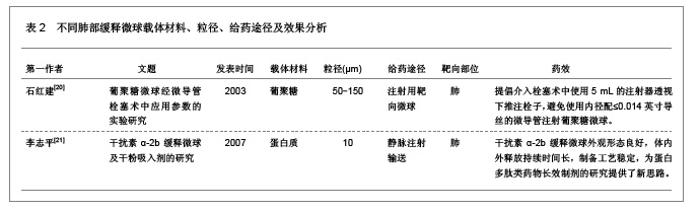
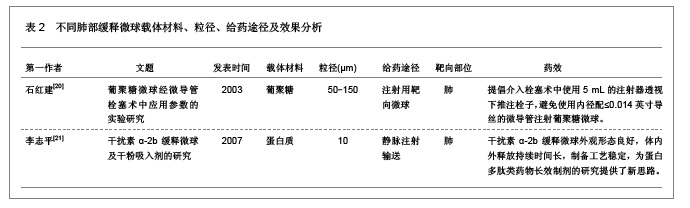
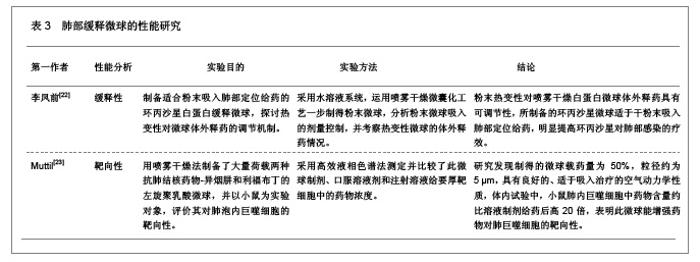
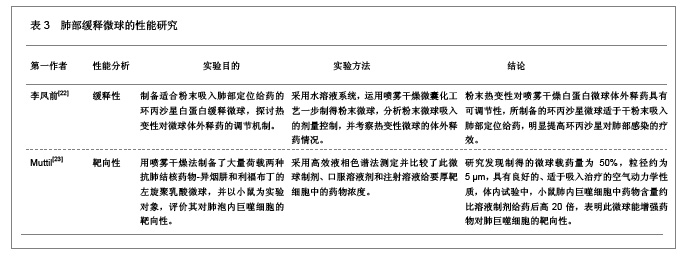
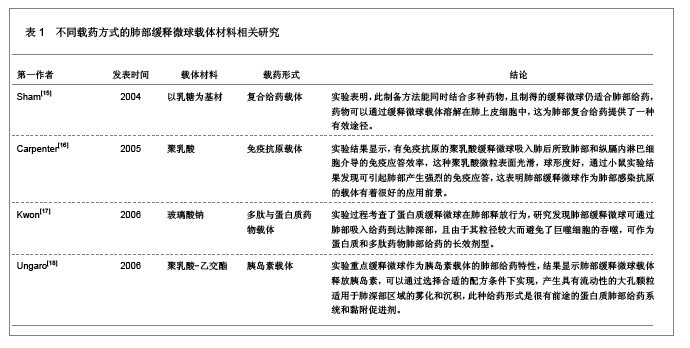
3 缓释微球治疗肺部疾病的特点及性能 3.1 资料来源 检索肺部缓释微球临床应用相关研究文献[13-14],检索时间范围1970至2012年,以“微球(Microspheres);药物控制释放(Controlled drug release);药物载体(Drug carrier);靶向性(Targeting);肺部给药(Pulmonary delivery);肺损伤(Lung injury);生物相容性(Biocompatibility);高分子材料(Polymer materials)”为检索词,选取文献36篇[15-50]。 3.2 纳入标准 ①肺部缓释微球缓释性、速释性和靶向性的研究。②肺靶向微球理化性质的研究。③肺部缓释微球的制备及性质的研究。 3.3 排除标准 ①存在具有临床意义的实验室检查异常者。②重复研究的文章。③排除较陈旧的理论观点。 3.4 分析指标 ①肺部缓释微球载体不同载药方式的应用研究。②肺部缓释微球载体材料的粒径及给药途径与药效的关系。③肺部缓释微球的优越性。④影响肺部缓释微球质量和体外释药的因素。 3.5 不同载药方式的肺部缓释微球载体材料 首先对于某些肺部疾病,单纯一种药物不足以使其治愈,需要两种或者多种药物同时作用,而将这种组成复方的药物通过肺部缓释微球载体携带实现了同时到达病灶部位起效的作用。其次,多肽与蛋白质药物通过口服给药因胃肠道生物酶的作用会导致药物生物利用度降低,而通过肺部缓释微球作为多肽和蛋白质药物载体避免生物酶的降解作用,并且肺部具有很大的吸收面积,使其成为极具应用价值和前景的非注射给药途径,不同载药方式的肺部缓释微球载体材料相关研究,见表1。 3.6 肺部缓释微球载体材料的粒径及给药途径与药效的关系 传统给药方式存在着给药后容易出现血药浓度峰谷现象及引起对胃肠道和心脏不良反应的毒副作用问题,使得开发新型的药物释放体系来代替传统药物体系,提高药物的有效利用率、减少对身体的危害成为药剂学发展的重要方向。肺部缓释微球可以维持较平稳的血药浓度,减少全身不良反应,延长药物作用时间,提高患者的顺应性。因此,运用生物降解材料及控释技术与呼吸临床治疗技术紧密结合,探索一种新的控释技术和剂型已成为目前研究的热点。用于肺部给药的微球制剂常用的材料有淀粉、聚乳酸、壳聚糖等。同时,微球用作靶向药物载体时,因粒径范围不同,靶向作用部位也不同[19]。常见的肺部缓释微球载体材料、微球粒径、给药途径与效果的关系,见表2。 3.7 肺部缓释微球的性能研究 载药肺部缓释微球通过雾化或干粉形式吸入,直接将药物运送至肺局部起效,进入肺部后沉积于肺部黏膜,释放药物,引起局部或全身作用,用于全身治疗的药物也可以通过此途径给药传递到肺泡区域,从而通过上皮细胞层而被吸收进入全身循环,可减少肺部疾病的药物用量,是治疗肺部疾病最理想的给药途径。肺部缓释微球具有增加药物溶解度、提高药物生物利用度、降低药物刺激性和延缓药物释放、增强靶向性与皮肤渗透性等作用,其最大的优点就是缓释、长效的同时,不会明显增加毒性,由于靶向性而降低毒副作用。因此,对载药微球理化性能的改善可以实现肺部给药的缓释性、靶向性等,提高载药量和药物生物利用度,也可以改善对大分子药物的传输性能。肺部缓释微球的性能研究,见表3。"
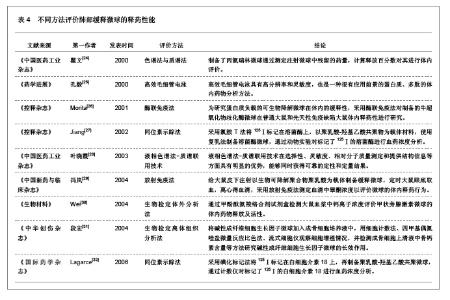
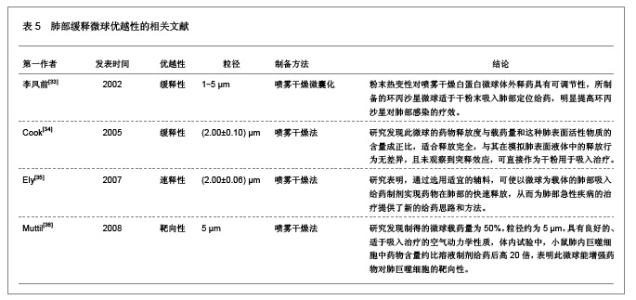
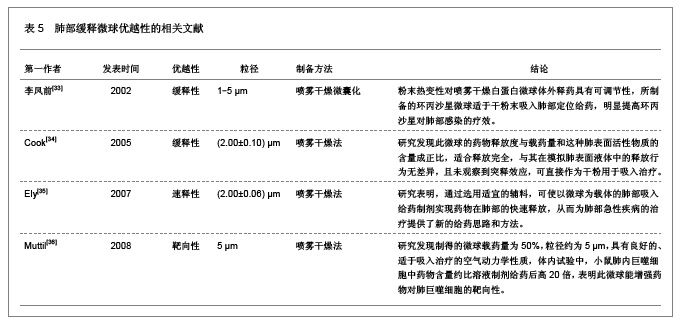
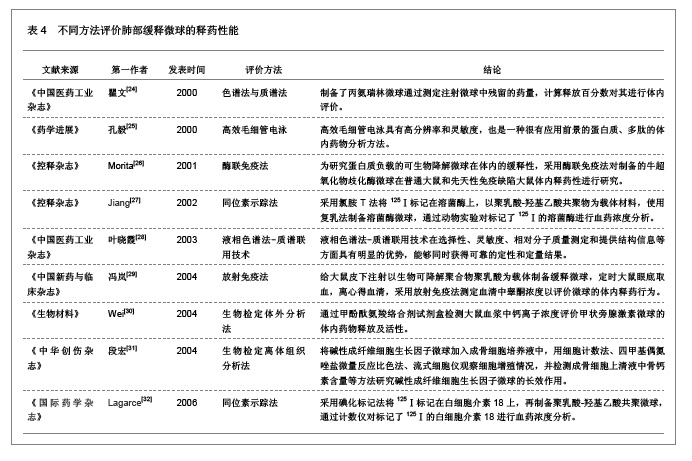
3.8 不同方法评价肺部缓释微球的释药性能 与传统药物制剂相比,微球制剂具有能及时释放药物、维持较高的供血药浓度或靶器官浓度,给药途径多样化,疗效持久、安全等优点。因此,利用微球作为载体的应用不仅能克服传统药物制剂的缺点,还能更好的发挥药物疗效,提高药物的生物活性和生物利用度。目前,利用微球释药的缓释性以及其与某些细胞组织的特殊亲和性,微球已广泛应用于抗癌、抗肿瘤制剂中。选用正确的体内药物分析方法是微球缓释性能研究的关键,结合微球剂型自身的特点,采用不同体内药物分析方法评价肺部缓释微球释药性的相关文献,见表4。 3.9 不同制备方法制备的肺部缓释微球的特点 肺部缓释微球能直接将药物输送至肺局部起效,可减少治疗肺部疾病的药物用量,并减小药物的全身毒副作用,是治疗肺部疾病的理想剂型,用于全身治疗的药物也可以通过肺部给药传递到肺泡区域,从而通过很薄的上皮细胞层而被吸收进入全身循环。肺部缓释微球优越性的相关文献,见表5。"

4 影响肺部缓释微球质量和体外释药的因素 聚合物的影响:Rahman[37]通过研究表明不同比例的聚乳酸-羟基乙酸共聚物和聚乳酸混合物制备的双层微球,突释效应具有显著差异,因此提示聚合物的浓度,相对分子质量大小和组成都会影响微球的体外释药。 微球粒径大小的影响:Görner等[38]在研究利多卡因聚乳酸纳米粒优化制备工艺时发现,大粒子载药量高,体外释放缓慢,小粒子释放时间短,因此,药物的释放速度与其在聚合物基质中的状态有关,但只要微球粒径小于300 μm,药物释放速度就与直径无关。 药物的影响:Elkheshen等[39]通过研究发现药物性质显著影响微球聚合物交联的稳定性,认为家养氯普安分子中的氨基和聚合物中自由羧基形成氢键或盐是聚合物载药量高于预期值的原因之一,药物和聚合物之间存在着相互作用。 释放介质及pH值的影响:Yang等[40]研究表明,重组人γ-干扰素聚乳酸共乙醇酸微球在pH值为5.0的琥珀酸盐缓冲液中释放最快,其次是在加入pH值为7.0的磷酸盐缓冲液中,因此认为释放介质的pH值将直接影响微球的药物释放。 5 结论 肺部因其特殊的生理结构而适合作为局部或全身用药的给药部位,而肺部缓释微球给药用以肺部局部疾病治疗以及全身治疗方面显示了独特的优越性。可以根据临床需要,通过加入不同辅料、采用不同制备方法来改进微球的结构和性能,使不同种类的微球进入肺部后能实现其靶向性、缓释性。目前已有一些微球制剂用于临床。国内外报道最多是微球的动脉栓塞化疗,该法已成为治疗某些肝肿瘤、肺肿瘤的首选方法。一些皮下植入或肌内注射的长效制剂也已用于临床。另外,治疗风湿性关节炎的关节腔内给药微球,蛋白质、多肽类生物活性大分子药物的鼻腔给药微球等已在动物实验方面取得重大突破。因此,随着医学、分子生物学、物理化学的发展以及药学工作者的共同努力,缓释微球制剂必将拥有广阔的应用前景。 作者贡献:王世寿进行实验设计,王世寿进行实验实施及评估,资料收集为王世寿、武刚,王世寿成文,王世寿对实验进行审校并对文章负责。 利益冲突:课题未涉及任何厂家及相关雇主或其他经济组织直接或间接的经济或利益的赞助。 伦理要求:实验获得所在单位的伦理委员会批注,符合伦理学标准。 作者声明:文章为原创作品,数据准确,内容不涉及泄密,无一稿两投,无抄袭,无内容剽窃,无作者署名争议,无与他人课题以及专利技术的争执,内容真实,文责自负。"

| [1] 李广灿.我国15种恶性肿瘤10年发病趋势分析[J].肿瘤防治研究,1994,21(6):388.[2] Kulkarni RK,Pani KC,Neuman C,et al.Polylactic acid for surgical implants.Arch Surg.1976;93(5):839-843.[3] 张冬.靶向缓释聚丙交酯微球[D].黑龙江:黑龙江大学,2005:1-86.[4] 王军红.药物释放系统(DDS)的应用现状及发展对策[D].北京:中国人民解放军军事医学科学院,2005:1-88.[5] Laube BL,Georgopoulos A,Adams GK 3rd.Preliminary study of the efficacy of insulin aerosol delivered by oral inhalation in diabetic patients.JAMA.1993;269(16):2106-2109.[6] Jendle JH,Karlberg BE.Intrapulmonary administration of insulin to healthy volunteers.J Intern Med.1996;240(2):93-98.[7] Ferraz MP,Mateus AY,Sousa JC,et al.Nanohydroxyapatite microspheres as delivery system for antibiotics: release kinetics, antimicrobial activity, and interaction with osteoblasts.J Biomed Mater Res A.2007;81(4):994-1004.[8] von Eiff C,Bettin D,Proctor RA,et al.Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis.Clin Infect Dis.1997;25(5):1250-1251.[9] 张万国,蒋雪涛,朱才娟,等.肺靶向利福平聚乳酸微球的研究[J].药学学报,1998,33(1):442.[10] 张万国,胡晋红,蒋雪涛,等.聚乳酸相对分子量对利福平微球及其药物分布状态的影响[J].解放军药学学报,2001,17(2):59-61.[11] 丁红,邢桂琴,谢茵.阿霉素明胶微球的制备与特性研究[J].中国医院药学杂志,2000,20(7):387-389.[12] 崔一民,鲁云兰,王培玉.利福平白蛋白微球的研制[J].中国医院药学杂志,1999,19(3):131-132.[13] 中国知网.中国学术期刊总库[DB/OL].2012-08-10. https://www.cnki.net[14] SCI数据库.Web of Sciencevia ISI Web of Knowledge[DB/OL]. 2012-08-10.http://ip-science.thomsonreuters.com/mjl. [15] Sham JO, Zhang Y, Finlay WH,et al.Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung.Int J Pharm. 2004; 269(2):457-467.[16] Carpenter ZK, Williamson ED, Eyles JE.Mucosal delivery of microparticle encapsulated ESAT-6 induces robust cell-mediated responses in the lung milieu. Control Release. 2005;104(1):67-77. [17] Kwon MJ, Bae JH, Kim JJ,et al.Long acting porous microparticle for pulmonary protein delivery.Int J Pharm. 2007;333(1-2):5-9.[18] Ungaro F, De Rosa G, Miro A,et al.Cyclodextrins in the production of large porous particles: development of dry powders for the sustained release of insulin to the lungs.Eur J Pharm Sci.2006;28(5):423-432.[19] Jalil R,Nixon JR.Microencapsulation using poly (L-lactic acid) II: Preparative variables affecting microcapsule properties.J Microencapsul.1990;7(1):25-39.[20] 石红建,王杰,施海彬,等.葡聚糖微球经微导管栓塞术中应用参数的实验研究[J].南通医学院学报,2003,23(1):6-8.[21] 李志平.干扰素α-2b缓释微球及干粉吸入剂的研究[D].北京:中国人民解放军军事医学科学院,2007:1-135.[22] 李凤前,陆彬,杨红,等.汉防己甲素缓释微囊肺靶向给药系统的研究[J].药学学报,2001,36(3):220-223.[23] Muttil P,Kaur J,Kumar K,et al.Inhalable microparticles containing large payload of anti-tuberculosis drugs.Eur J Pharm Sci.2007;32(2):140-150.[24] 瞿文,陈庆华,赵瑞钦,等.丙氨瑞林生物可降解缓释微球注射剂的研究[J].中国医药工业杂志,2000,31(1):15.[25] 孔毅,吴如金,吴梧桐,等.高效毛细管电泳及其在蛋白质、多肽分析中的应用[J].药学进展,2000,24(4):204-208.[26] Morita T, Sakamura Y, Horikiri Y,et al.Evaluation of in vivo release characteristics of protein-loaded biodegradable microspheres in rats and severe combined immunodeficiency disease mice.J Control Release.2001;73(2-3):213-221.[27] Jiang G,Woo BH,Kang F,et al.Assessment of protein release kinetics, stability and protein polymer interaction of lysozyme encapsulated poly(D,L-lactide-co-glycolide) microspheres.J Control Release.2002;79(1-3):137-145.[28] 叶晓霞,俞雄.多肽药物分析方法研究进展[J]. 中国医药工业杂志,2003,34(7):357-361.[29] 冯岚,郭健新,平其能,等.亮丙瑞林缓释微球的研究[J].中国新药与临床杂志,2004,23(10):680-683.[30] Wei G,Pettway GJ,McCauley LK,et al.The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres.Biomaterials. 2004;25(2):345-352.[31] 段宏,王光林,裴福兴,等.碱性成纤维细胞生长因子缓释微球对成骨细胞作用的实验研究[J].中华创伤杂志,2004,20(2):89-92.[32] Lagarce F,Garcion E,Faisant N,et al.Development and characterization of interleukin-18-loaded biodegradable microspheres.Int J Pharm.2006;314(2):179-188.[33] 李凤前,胡晋红,朱全刚.干粉末吸入环丙沙星缓释微球的制备及其体外释药调控[J].中国药学杂志,2002,37(2):110-113.[34] Cook RO,Pannu RK,Kellaway IW.Novel sustained release microspheres for pulmonary drug delivery.J Control Release.2005;104(1):79-90.[35] Ely L,Roa W,Finlay WH,et al.Effervescent dry powder for respiratory drug delivery.Eur J Pharm Biopharm.2007;65(3): 346-353.[36] Kaur J,Muttil P,Verma RK,et al.A hand-held apparatus for "nose-only" exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. Eur J Pharm Sci.2008;34(1):56-65.[37] Rahman NA,Mathiowitz E.Localization of bovine serum albumin in double-walled microspheres.J Control Release. 2004;94(1):163-175.[38] Görner T,Gref R,Michenot D,et al.Lidocaine-loaded biodegradable nanospheres. I. Optimization Of the drug incorporation into the polymer matrix.J Control Release.1999; 57(3):259-268.[39] Elkheshen SA,Radwan MA.Sustained release microspheres of metoclopramide using poly(D,L-lactide-co-glycolide) copolymers.J Microencapsul.2000;17(4):425-435.[40] Yang J,Cleland JL.Factors affecting the in vitro release of recombinant human interferon-gamma (rhIFN-gamma) from PLGA microspheres.J Pharm Sci.1997;86(8):908-914. |
| [1] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [2] | Liu Yang, Gong Yi, Fan Wei. Anti-hepatoma activity of targeted Pluronic F127/formononetin nanocomposite system in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 526-531. |
| [3] | Li Li, Ma Li. Immobilization of lactase on magnetic chitosan microspheres and its effect on enzymatic properties [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 576-581. |
| [4] | Li Xinping, Cui Qiuju, Zeng Shuguang, Ran Gaoying, Zhang Zhaoqiang, Liu Xianwen, Fang Wei, Xu Shuaimei. Effect of modification of β-tricalcium phosphate/chitosan hydrogel on growth and mineralization of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3493-3499. |
| [5] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| [6] | Zhou Anqi, Tang Yufei, Wu Bingfeng, Xiang Lin. Designing of periosteum tissue engineering: combination of generality and individuality [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3551-3557. |
| [7] | Lang Limin, He Sheng, Jiang Zengyu, Hu Yiyi, Zhang Zhixing, Liang Minqian. Application progress of conductive composite materials in the field of tissue engineering treatment of myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3584-3590. |
| [8] | Ma Qing, Shi Liyan, Huang Sixue, Zheng Zhangbowen, Zhang Aihua, Zhan Desong, Fu Jiale. Research status and prospect of zirconia ceramics in dental prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3597-3602. |
| [9] | Wen Zhijing, Gu Pengzhen, He Xijing, Li Jialiang, Wang Yibin, Wang Yiqun. Development of high molecular polymer polyetherketoneketone and its prospects in medical applications [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3603-3608. |
| [10] | Liao Yuan, Qu Mengjian, Liu Jing, Zhong Peirui, Zeng Yahua, Wang Ting, Xiao Hao, Li Lan, Liu Danni, Yang Lu, Zhou Jun. Effects of ultrashort wave on inflammatory response in rats with acute lung injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 253-257. |
| [11] | Chen Siyu, Li Yannan, Xie Liying, Liu Siqi, Fan Yurong, Fang Changxing, Zhang Xin, Quan Jiayu, Zuo Lin. Thermosensitive chitosan-collagen composite hydrogel loaded with basic fibroblast growth factor retards ventricular remodeling after myocardial infarction in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2472-2478. |
| [12] | Liu Feng, Zhang Yu, Wang Yanli, Luo Wei, Han Chaoshan, Li Yangxin. Application of temperature-sensitive chitosan hydrogel encapsulated exosomes in ischemic diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2479-2487. |
| [13] | Li Jie, Xu Jianzhen, Hu Ping, Lei Qiqi, Zhang Wenning, Ao Ningjian . Preparation and performance evaluation of carboxymethyl chitosan/oxidized glucomannan/Panax notoginseng compound sponge dressing for chronic wound [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2528-2534. |
| [14] | Li Shao, Liang Yongkang, Gao Yi, Peng Qing. Establishment and functional in vitro characteristics of three-dimensional collagen HepaRG microsphere [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2541-2547. |
| [15] | Gan Zhoujie, Pei Xibo. Enzyme-responsive nanoparticles in tumor therapy: superiority of nanoparticles in accumulation and drug release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2562-2568. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||