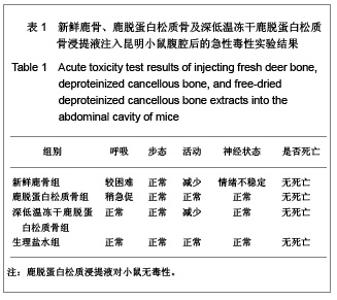
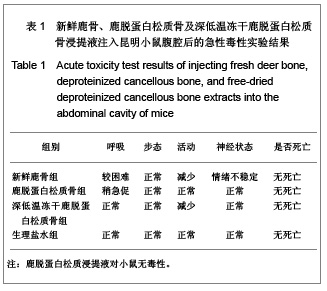
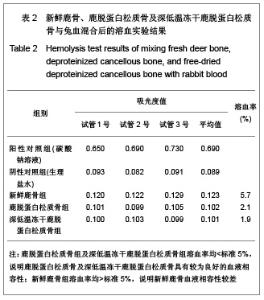
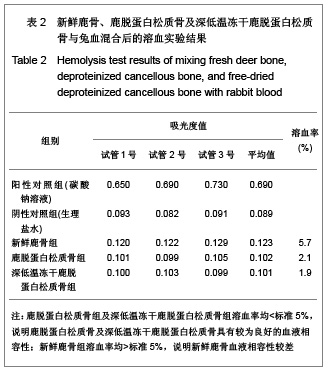
| [1] Seiler JG 3rd, Johnson J.Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc.2000;9(2):91-97.[2] Orban JM,Marfa KG,Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng.2002;8(4):529-539.[3] Boone DW. Complications of iliac crest graft and bone grafting alternatives in foot and ankle surgery. Foot Ankle Clin.2003;8(1):1-14. [4] Wang DC,Li K,Cui B,et al.Zuzhi Gongcheng yu Chongjian Waike Zazhi.2009;5(1):32-34. 王德超,李奎,崔冰,等.去抗原异种松质骨材料的生物相容性研究[J].组织工程与重建外科杂志,2009,5(1):32-34.[5] Bertollo N,Matsubara M,Shinoda T,et al.Effect of surgical fit on integration of cancellous bone and implant cortical bone shear strength for a porous titanium. J Arthroplasty. 2011;26(7):1000-1007.[6] Kadir MR,Syahrom A,Ochsner A. Finite element analysis of idealised unit cell cancellous structure based on morphological indices of cancellous bone.Med Biol Eng Comput. 2010;48(5):497-505. [7] van der Meulen MC, Yang X, Morgan TG, et al.The effects of loading on cancellous bone in the rabbit. Clin Orthop Relat Res. 2009;467(8):2000-2006.[8] Bagi CM, Berryman E, Moalli MR.Comparative bone anatomy of commonly used laboratory animals: implications for drug discovery.Comp Med.2011;61(1):76-85.[9] Missiuna PC, Gandhi HS, Farrokhyar F, et al.Anatomically safe and minimally invasive transcrestal technique for procurement of autogenous cancellous bone graft from the mid-iliac crest. Can J Surg.2011;54(5):327-332.[10] Sahibzada AS, Khan MA, Khan MS. Management of tibial bone defects due to high energy trauma using the locally manufactured external fixator by segmental bone transport. J Avub Med Coll Abbottabad.2005;17(3): 68-72.[11] Roshan-Ghias A, Arnoldi J, Procter P, et al.In vivo assessment of local effects after application of bone screws delivering bisphosphonates into a compromised cancellous bone site. Clin Biomech (Bristol, Avon).2011;26(10): 1039-1043.[12] Sawada Y,Hokugo A,Yang Y,et al.A novel hydroxyapatite ceramic bone substitute transformed by ostrich cancellous bone: characterization and evaluations of bone regeneration activity. J Biomed Mater Res B Appl Biomater. 2011;98B(2): 217-222. [13] Wang Y, Liu G, Li T, et al. Morphometric comparison of the lumbar cancellous bone of sheep, deer, and humans.Comp Med. 2010; 60(5): 374-379.[14] Schortinghuis J,Putters TF,Raghoebar GM. Safe harvesting of outer table parietal bone grafts using an oscillating saw and a Bone scraper: a refinement of technique for harvesting cortical and “cancellous”-Like calvarial bone.J Oral Maxillofac Surg.2012;70(4):963-965.[15] Gao CY,Jiang HC,Jin CM.Zhongguo Zuzhi Gongcheng Yanjiu yu Linchang Langfu. 2011;15(25):4661-3664. 高春阳,姜宏春,金春明.脱蛋白松质骨作为异种骨移植材料的修复作用[J].中国组织工程研究与临床康复,2011,15(25): 4661-3664.[16] Sugiyama T,Price JS,Lanyon LE,et al.Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones.Bone. 2010; 46(2): 314-321.[17] Sachlos E, Reis N, Ainsley C, et a.Novel collagen scaffolds with predefined internal morphology made by solid freedom fabrication.Biomaterals.2003;24(8):1487-1497.[18] Qu XY,Jiang DM,An H,et al.Zhongguo Chuangshang Zazhi. 2007;23(8):605-608. 瞿向阳,蒋电明,安洪,等.重组异体脱蛋白骨关节移植修复骨关节缺损的实验研究[J]中国创伤杂志,2007,23(8):605-608. |