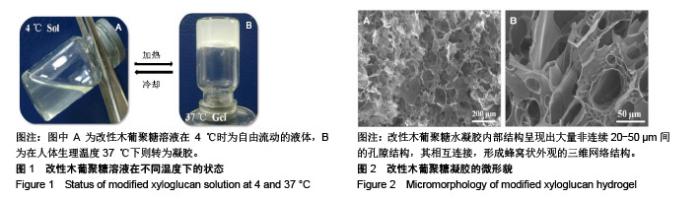
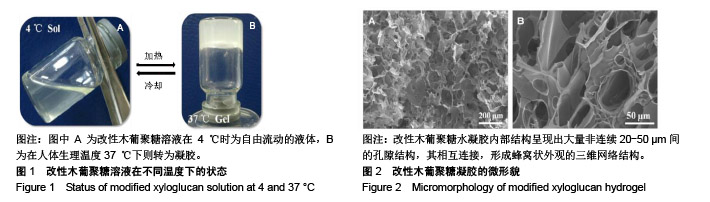
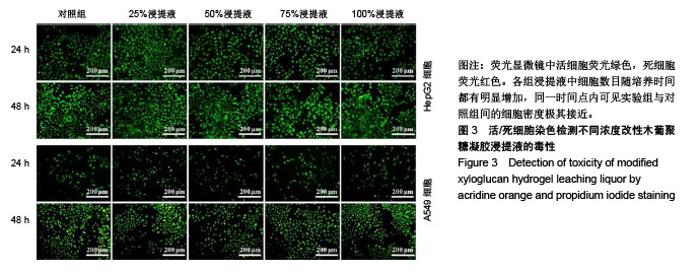
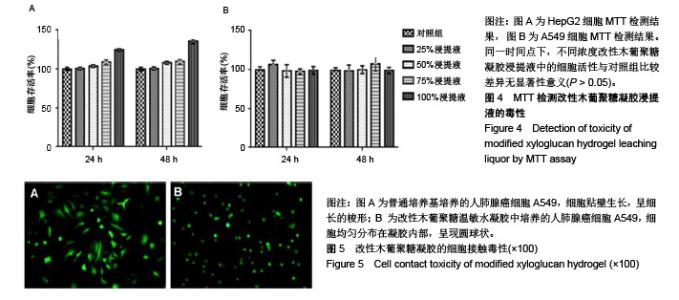
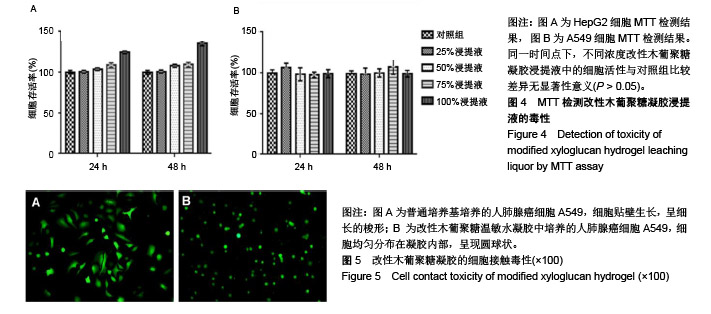
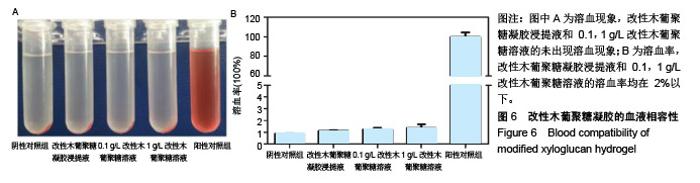
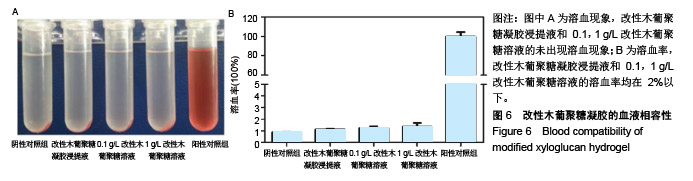
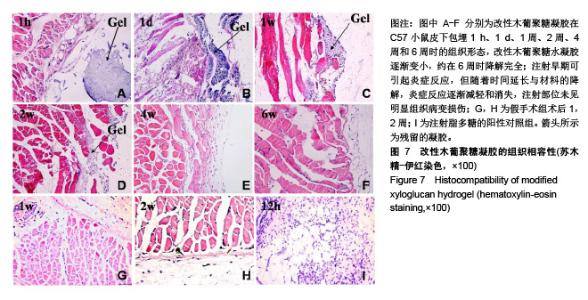
| [1]贾宗良,平高峰,王康,等.COX-2?VEGF在腹腔粘连组织中的表达及其意义[J].西安交通大学学报(医学版), 2010,31(5): 608-611.[2]Gong CY,Wu QJ,Liao JF,et al. Prevention of postsurgical cauterization-induced peritoneal adhesions by biodegradable and hermosensitive micelles.J Biomed Nanotechnol.2013;9(12):1984-1995.[3]Yeo Y,Highley CB,Bellas E,et al.In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials.2006;27(27): 4698-4705.[4]Li L,Wang N,Jin X. Biodegradable and injectable in situ crosslinking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention.Biomaterials.2014; 35(12):3903-3917.[5]Park KS,Lee KE,Ku do H,et al.Antiadhesive effect and safety of oxidized regenerated cellulose after thyroidectomy: a prospective, randomized controlled study.J Korean Surg Soc.2013;84(6):321-329.[6]林思,秦飞,宋路瑶,等.丹参酮ⅡA磺酸钠通过增强腹膜纤溶系统活性降低大鼠术后腹膜粘连发生[J].南方医科大学学报, 2016,36(2):260-264.[7]Cai L,Dewi RE,Heilshorn SC.Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells .Advanced Functional Materials. 2015;25(9):1344-1351.[8]Okabayashi K,Ashrafian H,Zacharakis E,et al. Adhesions after abdominal surgery: a systematic review of the incidence, distribution and severity.Surg Today. 2014; 44(3):405-420.[9]Diamond MP,Wexner SD,diZereg GS,et al.Adhesion prevention and reduction:current status and future recommendations of a multinational interdisciplinary consensus conference.Surg Innov.2010;17(3):183-188.[10]Zhang H,Song Y,Li Z,et al.Evaluation of breviscapine onprevention of experimentally induced abdominal adhesions in rats.Am J Surg.2016;211(6):1143-1152.[11]崔菁,张亚彬,马思琪,等.卡拉胶/聚乙烯醇复合材料的制备及生物相容性评价[J].中国组织工程研究,2017,21(2):215-220.[12]Yigitler C,Karakas DO,Kucukodaci Z,et al. Adhesion-preventing properties of 4% icodextrin and canola oil:acomparative experimental study. Clinics.2012;67(11): 1303-1308.[13]Wei G,Chen X,Wang G,et al.Effect of Resveratrol on the Prevention of intra-abdominal adhesion formation in a rat model.Cell Physiol Biochem.2016;39(1):33-46.[14]Brochhausen C,Schmitt VH,Planck CN,et al.Current strategies and future perspectives for intraperitoneal adhesion prevention.J Gastrointest Surg. 2012;16(6): 1256-1274.[15]Bothin C,Okada M,Midtvedt T,et al.The intestinal flora influences adhesion formation around surgical anastomoses.Br J Surg.2001;88(1):143-145.[16]Bae SH,Son SR,Kumar Sakar S,et al.Evaluation of the potential anti-adhesion effect of the PVA/Gelatin membrane.J Biomed Mater Res B Appl Biomater. 2014; 102(4):840-849.[17]Moon HJ,Ko du Y,Park MH,et al.Temperature-responsive compounds as in situ gelling biomedical materials.Chem Soc Rev.2012;41(14):4860-4883.[18]Zhang Y,Gao C,Li X,et al.Thermosensitive methyl cellulose-based injectable hydrogels for post-operation anti-adhesion.Carbohydr Polym.2014;101(1):171-178.[19]Zheng Y, Liang Y, Zhang D,et al. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega. 2018;3(5):4766-4775.[20]高萍,程琳,陈斌,等.医用可注射羧甲基葡糖胺聚糖防粘连凝胶的生物安全性评价[J].中国组织工程研究, 2019,23(10): 1570-1574.[21]Bülbüller N,Karaka? BR,Y?ld?r?m HT,et al. Effect of a new cross-linked hyaluronan gel on the staple line after sleeve gastrectomy in a rat model. Acta Cirurgica Brasileira. 2018; 33(2):163-174.[22]何楠,龙安妮,胡畅,等.聚乙烯醇-羧甲基纤维素钠复合膜预防血管开放术后黏连的实验研究[J].中国循环杂志, 2018,33(7): 714-718.[23]Albiñanacunningham JN,Ripaldacemboráin P,Labiano T,et al.Mechanical barriers and transforming growth factor beta inhibitor on epidural fibrosis in a rabbit laminectomy model. J Orthop Surg Res. 2018;13(1):72.[24]Montalvo-Javé EE,Mendoza-Barrera GE,Garcí-A-Pineda MA,et al.Histological Analysis of Intra-Abdominal Adhesions Treated with Sodium Hyaluronate and Carboxymethylcellulose Gel. J Invest Surg.2016;29(2):80-87.[25]曾德强,李治国,朱晓峰,等.新型疝修补材料修补新西兰大白兔腹壁缺损的效果[J].广东医学,2014,35(7):998-1001.[26]连紫宇,杨丽丽,卞尧尧,等.川芎嗪纳米喷雾剂干预术后腹腔粘连[J].中国组织工程研究,2018,22(18):2896-2902.[27]Dhall S, Coksaygan T, Hoffman T, et al. Viable cryopreserved umbilical tissue (vCUT) reduces post-operative adhesions in a rabbit abdominal adhesion model. Bioact Mater. 2018;4(1):97-106.[28]池余刚,杨晓煜,何萍,等.经皮雌二醇凝胶用于中重度宫腔粘连术后辅助治疗的研究[J].生殖医学杂志,2016,25(8):691-695. |