Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (4): 644-649.doi: 10.3969/j.issn.2095-4344.0104
Previous Articles Next Articles
Effects of skeletal muscle satellite cells on muscle fiber lesions
Huang He-ping1, Xu Shuang1, Xiao Tao-fang2, Lei Xiao-feng3
- 1Sport College, Gannan Normal University, Ganzhou 341000, Jiangxi Province, China; 2Nanchang Institute of Science and Technology, Nanchang 330108, Jiangxi Province, China; 3Affiliated Middle School of Gannan Normal University, Ganzhou 341000, Jiangxi Province, China
-
Received:2017-08-26Online:2018-02-08Published:2018-02-08 -
About author:Huang He-ping, Studying for doctorate, Associate professor, Sport College, Gannan Normal University, Ganzhou 341000, Jiangxi Province, China
CLC Number:
Cite this article
Huang He-ping1, Xu Shuang1, Xiao Tao-fang2, Lei Xiao-feng3. Effects of skeletal muscle satellite cells on muscle fiber lesions[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(4): 644-649.
share this article
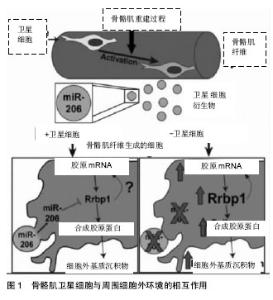
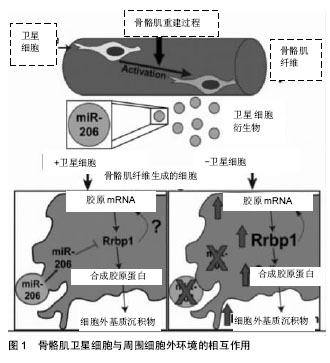
2.1 骨骼肌卫星细胞功能的调控 卫星细胞独立于骨骼肌,位于细胞膜和肌膜之间,是具有增殖分化潜力的肌源性干细胞,主要负责骨骼肌纤维的损伤修复和生长[1,15]。正常情况下,骨骼肌卫星细胞处于静止状态,当肌肉受到损伤刺激后,卫星细胞将会被激活,进行有丝分裂、基因表达和增殖分化,形成骨骼肌干细胞[16-17]。 骨骼肌卫星细胞可以提供骨骼肌修复的成肌细胞,具有维持、修复骨骼肌结构的功能。通过运动训练可以有效激活骨骼肌卫星细胞,在运动训练中主要通过骨骼肌的微损伤、炎性因子释放、局部生长因子和细胞因子的释放增多等途径激活骨骼肌卫星细胞,然后通过基因表达、增殖、分化形成骨骼肌干细胞达到维持和修复骨骼肌的形态结构和功能的作用[18-22]。长期力量练习可有效增加骨骼肌卫星细胞的数量,研究表明,经过多年训练的优秀举重运动员斜方肌卫星细胞高于对照组的70%。因此,骨骼肌的损伤修复和再生取决于静息状态的卫星细胞的激活数目以及被激活卫星细胞的分裂增殖能力。 骨骼肌卫星细胞与周围细胞外环境的相互作用可促进组织可塑性,见图1。Fry[23]的研究团队报道骨骼肌肌源性祖细胞分泌含有含有miR-206的外泌体(脊椎动物特有的miRNA),通过抑制核糖体结合蛋白(Rrbp1) 调节纤维形成细胞胶原表达。骨骼肌肌源性祖细胞外泌体在肌肉生理性肥大过程中发挥防止细胞外基质的过度沉积、促进长期肌纤维肥大的重要作用。"
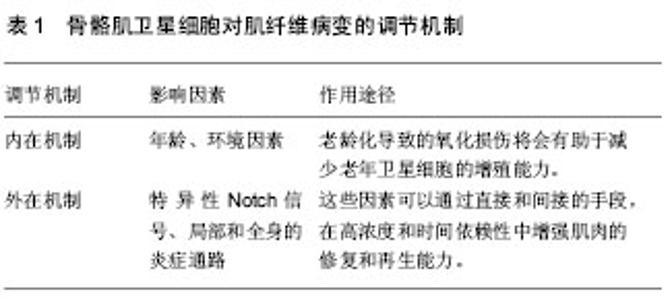
骨骼肌卫星细胞功能的适当控制是有效调节肌肉再生的关键,也是有效防止肌肉肿瘤出现的途径之一。一定数目的通路涉及到调节肌卫星细胞的再生功能。骨骼肌卫星细胞在特异表达的过程中,会出现一些标志蛋白,这些蛋白与骨骼肌卫星细胞的再生功能的调节有关,包括白细胞介素6、胰岛素生长因子、成纤维细胞生长因子、机械生长因子和转化生长因子β,以及局部的炎症介质[24-27]。研究证实,体外的细胞培养研究中发现胰岛素生长因子Ⅰ和胰岛素生长因子Ⅱ可以促进卫星细胞增殖。在损伤肌肉的肌膜下注射外源性胰岛素生长因子Ⅰ,可以促进卫星细胞增殖,出现骨骼肌纤维肥大。此外,非肌细胞在调节卫星细胞增殖和分化中所起的作用也备受瞩目[28]。与骨骼肌卫星细胞的功能调控相关的非肌细胞,构成了异构混合的间充质细胞类型,包括脂肪组织、纤维组织以及浸润造血的细胞类型。 2.2 肌肉疾病与肌肉失用对骨骼肌卫星细胞功能的影响 肌肉失用性萎缩是肢体在制动、固定及其失重状态下发生的生理、生化、形态学及功能的变化。许多病理条件下,包括先天性肌病在内的神经和肌肉,由于失用性萎缩,所涉及的肌肉质量和力量逐渐丧失,表现出一定数量的肌卫星细胞的增殖潜能减弱[29]。研究表明,肌肉失用可以导致骨骼肌卫星细胞的数目减少。而长期的运动训练可以有效的维持卫星细胞池以及增加骨骼肌卫星细胞的数目,促进卫星细胞的自我更新[30-34]。研究表明,在运动员停训后骨骼肌卫星细胞数目开始逐渐减少,直至出现卫星细胞活化终止[35]。总之,主要基因病变引起肌肉营养不良(受卫星细胞影响相关的疾病)和肌肉失用,这些将会导致渐进性肌肉病变。 研究表明,骨骼肌卫星细胞的激活是肌肉再生和修复的前提[36-37]。在运动损伤后,静息状态的卫星细胞被激活。在骨骼肌卫星细胞增殖分化后,可以达到细胞核增多、RNA转录、肌管系统生成和肌原纤维增多的效果。由于慢性增殖和潜在的抑制性微环境,可以导致卫星细胞数量减少,并加速破坏疾病或营养不良的肌肉组织平衡。现有研究表明,肌营养不良可导致骨骼肌卫星细胞再生功能过早丧失[38-39]。在肌营养不良症的小鼠模型中,骨骼肌卫星细胞数量的减少可导致慢性退行性条件下的重复性肌肉再生障碍。重复周期性的卫星细胞激活可导致端粒缩短或者关键卫星细胞调控的基因突变,使骨骼肌卫星细胞的自我更新活性受损和肌源性容量的受损,加剧了骨骼肌得营养不良,卫星细胞增殖缺乏和再生潜能的受损。 2.3 老龄化与肌肉功能对骨骼肌卫星细胞修复能力的影响 老龄化导致的肌肉功能下降与骨骼肌微环境营养的缺乏有密切的关系。由于骨骼肌卫星细胞的内在缺陷,导致在骨骼肌卫星细胞功能改变的过程中,营养的缺乏可以抑制这些细胞的肌源性活动。另外,一些非肌源性细胞类型不能促进肌肉再生,例如,在营养不良的患者中成纤维细胞所分泌的胰岛素样生长因子结合蛋白水平增加,将会隔离胰岛素样生长因子结合蛋白远离肌源性细胞[40]。同样,分泌的细胞因子,包括细胞增殖、迁移,诱导基质细胞衍生因子1α,可以诱发引起损伤骨骼肌的修复和再生,可以帮助调节和组织新骨骼肌的肌生成。通过这些观察和研究,结果表明,卫星细胞微环境对调节骨骼肌前体细胞活性具有关键性的作用。 年龄的增加,骨骼肌纤维数量的减少(以Ⅱ型肌纤维为主),出现肌肉力量下降,即骨骼肌减少症,这将严重影响到骨骼肌的机能和运动能力。卫星细胞数目和功能的减少,根源在于年龄增大出现的肌肉病理所导致。许多研究已经记录了特定年龄相关的肌肉维护和再生的不足,研究表明,肌纤维相关卫星细胞数下降与年龄有关,活性肌卫星细胞老化的增殖能力明显受损,会引起更快速的进入衰老[41-42]。然而,在适当的条件下,卫星细胞的增殖能力以通过一定程度耐力运动得到恢复或保持。在运动的过程中,肌纤维出现损伤,骨骼肌卫星细胞将被激活,随后出现增殖、分化,融合成新的骨骼肌纤维以修复损伤的肌纤维或肌细胞核。目前的研究表明,运动训练可以诱导骨骼肌质量和肌细胞核的增加,同时伴随着卫星细胞数量和激活状态的增加。 2.4 骨骼肌卫星细胞对肌纤维病变的调节机制 骨骼肌卫星细胞对肌纤维病变的调节机制可以分为内在机制和外在机制,见表1。"

| [1] Mourikis P, Sambasivan R, Castel D, et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243-252. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [12] | Yu Langbo, Qing Mingsong, Zhao Chuntao, Peng Jiachen. Hot issues in clinical application of dynamic contrast-enhanced magnetic resonance imaging in orthopedics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 449-455. |
| [13] | Bai Shengchao, Gao Yang, Wang Bo, Li Junping, Wang Ruiyuan. Dynamic changes of mitochondrial function of the skeletal muscle after acupuncture intervention in rats with heavy load exercise-induced injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3648-3653. |
| [14] | Wang Zhen, Lin Haiqi, He Fei, Lin Wentao. Exercise activates skeletal muscle satellite cells: exercise prevention and treatment for age-related sarcopenia and muscle injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3752-3759. |
| [15] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 79
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 449
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||