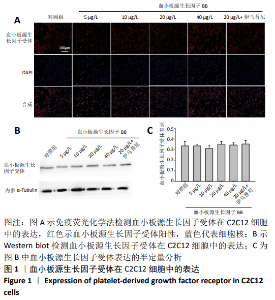
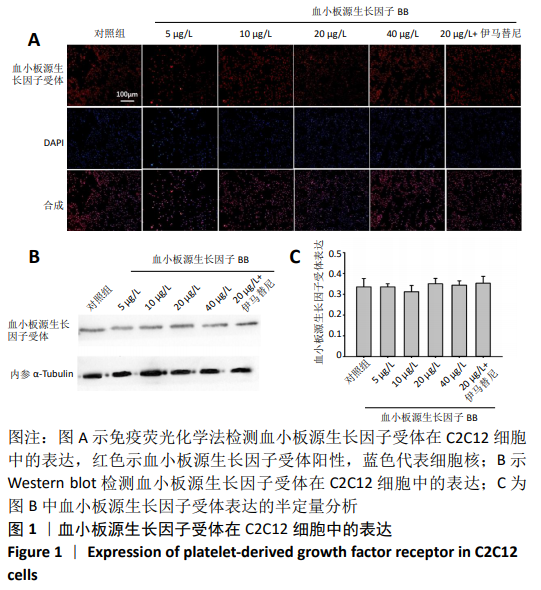
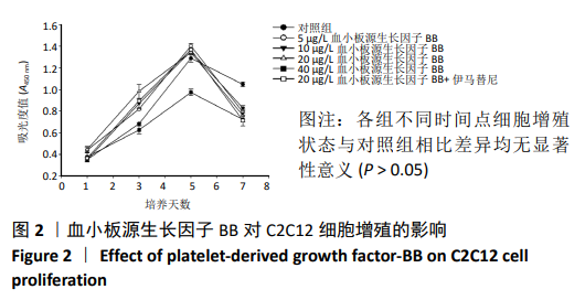
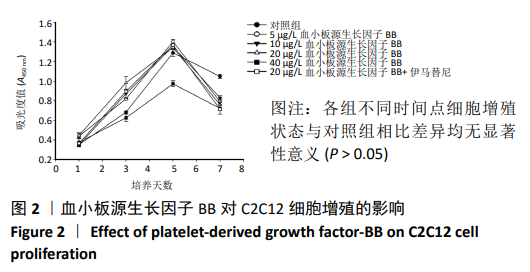
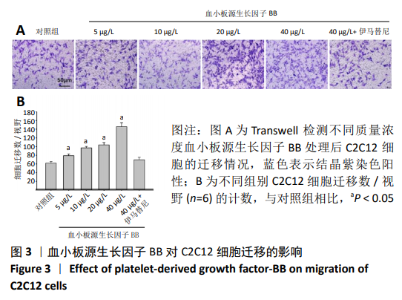
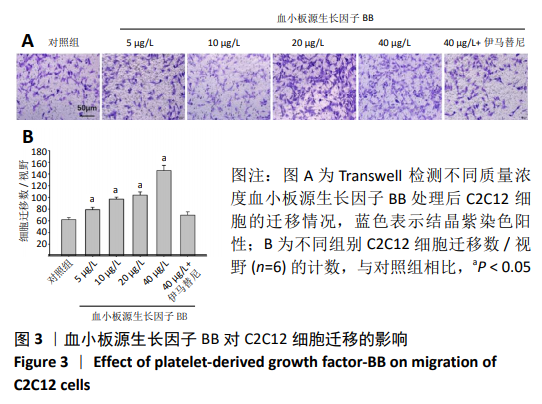
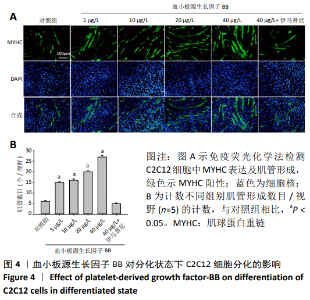
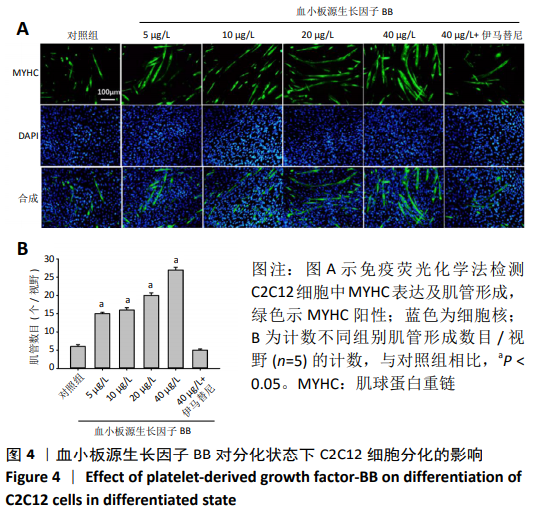
[1] LEHTO MU, JÄRVINEN MJ.Muscle injuries, their healing process and treatment. Ann Chir Gynaecol. 1991;80(2):102-108.
[2] TANG X,DANESHMANDI L,AWALE G,et al. Skeletal muscle regenerative engineering. Regen Engd Transl Med. 2019: 1-19.
[3] 刘晓光,赵淋淋,曾志刚,等.小鼠骨骼肌损伤修复过程中肌再生调节因子和血管再生因子的表达规律研究[J].中国康复医学杂志, 2016,31(12) : 1294-1300.
[4] 王大鹏,张桂梅,罗力佳.细胞生长因子修复骨骼肌损伤[J].中国组织工程研究,2016,20(2): 273-278.
[5] TIDBALL JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol. 2011;1(4):2029-2062.
[6] 丁维进,苏志达,江华.肌源干细胞在组织工程和再生医学的应用[J].中国组织工程研究,2013,17(23): 4328-4333.
[7] 袁启龙,曾晓勇,陈亮,等.兔不同部位脂肪来源干细胞体外成肌诱导能力的比较[J]. 中国修复重建外科杂志,2010,24(10): 1228-1232.
[8] YIN H, PRICE F, RUDNICKI MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23-67.
[9] NEELAKANTAN H, BRIGHTWELL CR, GRABER TG, et al. Small molecule nicotinamide N-methyltransferase inhibitor activates enescent muscle stem cells and improves regenerative capacity of aged skeletal muscle . Biochem Pharmacol. 2019;163:481-492.
[10] ZHAO Y, CHEN M, LIAN D, et al. Non-Coding RNA Regulates the Myogenesis of Skeletal Muscle Satellite Cells, Injury Repair and Diseases. Cells. 2019;8(9):988.
[11] KHARRAZ Y, GUERRA J, PESSINA P, et al. Understanding the process of fibrosis in Duchenne muscular dystrophy . Biomed Res Int. 2014; 2014:965631.
[12] PRASAD A, LIN F, CLARK RAF. Fibronectin-derived Epiviosamines enhance PDGF-BB-stimulated human dermal fibroblast migration in vitro and granulation tissue formation in vivo. Wound Repair Regen. 2019.
[13] WANG C, LIU Y, HE D. Diverse effects of platelet-derived growth factor-BB on cell signaling pathways . Cytokine. 2019;113:13-20.
[14] 裴丹,李洪鹏. PDGFR/PI3K/Akt信号通路在PDGF-BB 促进体外小鼠脑星形胶质细胞增殖中的作用[J].中国医科大学学报,2019,48(12): 1076-1081.
[15] WANG F, HOU K, CHEN W, et al. Transgenic PDGF-BB/sericin hydrogel supports for cell proliferation and osteogenic differentiation. Biomater Sci. 2020;8(2):657-672.
[16] GAO SY, LIN RB, HUANG SH, et al. PDGF-BB exhibited therapeutic effects on rat model of bisphosphonate-related osteonecrosis of the jaw by enhancing angiogenesis and osteogenesis. Bone. 2019;29:115117.
[17] DENG J, PAN J, HAN X, et al. PDGFBB-modified stem cells from apical papilla and thermosensitive hydrogel scaffolds induced bone regeneration. Chem Biol Interact. 2020;316:108931.
[18] DAS S, MAJID M, BAKER AB. Syndecan-4 enhances PDGF-BB activity in diabetic wound healing. Acta Biomater. 2016;42:56-65.
[19] CAPPELLARI O, BENEDETTI S, INNOCENZI A,et al. Dll4 and PDGF-BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev Cell. 2013;24(6):586-599.
[20] BAGHDADI MB, TAJBAKHSH S. Regulation and phylogeny of skeletal muscle regeneration. Dev Biol. 2018;433(2):200-209.
[21] REUTERDAHL C, SUNDBERG C, RUBIN K, et al. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derivedgrowth factor B chain during wound repair in humans. J Clin Invest. 1993;91(5):2065-2075.
[22] MAJESKY MW, REIDY MA, BOWEN-POPE DF, et al. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990;111(5 Pt 1):2149-2158.
[23] SMITH CW, KLAASMEYER JG, WOODS TL, et al. Effects of IGF-I, IGF-II, bFGF and PDGF on the initiation of mRNA translation in C2C12myoblasts and differentiating myoblasts. Tissue Cell. 1999;31(4): 403-412.
[24] PAPADOPOULOS N, LENNARTSSON J. The PDGF/PDGFR pathway as a drug target. Mol Aspects Med. 2018;62:75-88.
[25] 王媚媚,秦晓红,米立志.血小板衍生生长因子受体结构与功能的研究[J].中国科学: 生命科学, 2019,49(6):683-697.
[26] ZANOU N, GAILLY P.Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatoryfactors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 2013;70(21): 4117-4130.
[27] SCULLY D, SFYRI P, VERPOORTEN S, et al. Platelet releasate promotes skeletal myogenesis by increasing muscle stem cell commitment to differentiation and accelerates muscle regeneration following acute injury. Acta Physiol (Oxf). 2019;225(3):e13207.
[28] SALHA S, GEHMERT S, BRÉBANT V, et al. PDGF regulated migration of mesenchymal stem cells towards malignancy acts via the PI3K signaling pathway. Clin Hemorheol Microcirc. 2018;70(4):543-551.
[29] WANG DF, LIU Y, WANG J. rhPDGF-BB promotes migration of bone marrow stromal stem cells in diabetic rats. Shanghai Kou Qiang Yi Xue. 2018;27(6):579-584.
[30] PIRAN M, VAKILIAN S, PIRAN M, et al. In vitro fibroblast migration by sustained release of PDGF-BB loaded in chitosan nanoparticles incorporated in electrospun nanofibers for wound dressing applications. Artif Cells Nanomed Biotechnol. 2018;46(sup1):511-520.
[31] KOVACEVIC D, GULOTTA LV, YING L, et al . rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res. 2015; 473(5):1644-1654. |