Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (4): 621-626.doi: 10.12307/2023.959
Previous Articles Next Articles
Mechanism of negative pressure wound therapy in the auxiliary treatment of bone and soft tissue infection
Xing Hao1, 2, Meng Qingfeng3, Chang Zhengqi2
- 1School of Clinical Medicine, Weifang Medical University, Weifang 261053, Shandong Province, China; 2Department of Orthopedics, the 960th Hospital of the PLA Joint Logistics Support Force, Jinan 250031, Shandong Province, China; 3Shandong Yellow River Hospital, Jinan 250032, Shandong Province, China
-
Received:2022-12-13Accepted:2023-02-14Online:2024-02-08Published:2023-07-14 -
Contact:Chang Zhengqi, MD, Associate chief physician, Department of Orthopedics, the 960th Hospital of the PLA Joint Logistics Support Force, Jinan 250031, Shandong Province, China -
About author:Xing Hao, Master candidate, Physician, School of Clinical Medicine, Weifang Medical University, Weifang 261053, Shandong Province, China
CLC Number:
Cite this article
Xing Hao, Meng Qingfeng, Chang Zhengqi. Mechanism of negative pressure wound therapy in the auxiliary treatment of bone and soft tissue infection[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 621-626.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

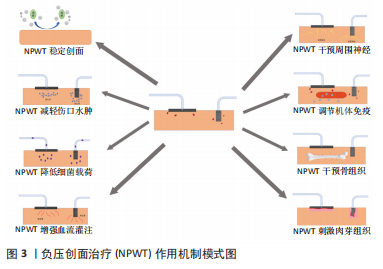
2.1 NPWT稳定创面 创面环境的复杂性在一定程度上影响创面的愈合状况,如严重污染或暴露的创面更易发生细菌定植感染导致愈合时间延长,引起创面愈合不良;清洁创面不仅减轻患者在愈合过程中的痛苦,而且显著缩短患者住院时间。NPWT可趋化引流细菌及清除部分炎症递质和细胞因子,促进创面愈合。在骨与软组织感染治疗中,NPWT不但将大部分细菌和坏死组织通过负压引流到密封的漏瓶中,从而保持感染创面的相对清洁,而且可以明显降低细菌、毒素和炎症因子吸收入血,防止菌血症、败血症的发生,改善患者的全身状况,为后续治疗奠定基础[5]。 NPWT可有效隔离创面与外界环境,并通过泡沫敷料将负压均匀分布在接触面上。泡沫敷料种类因创面不同而有所差异,目前常见的泡沫敷料主要是黑色的聚氨酯和白色的聚乙烯醇。聚氨酯材料的泡沫孔径较大,疏水性好,适用于渗液多或有大量肉芽组织形成的创面,但不适用于深部的创腔及窦道。临床上使用的聚氨酯泡沫敷料部分为含银聚氨酯泡沫塑料,主要用于严重污染的创面。聚乙烯醇材料的泡沫孔隙更致密,亲水性好,组织相容性高,具有更高的抗拉强度且可以限制肉芽组织形成,减轻换药的疼痛,因而适用于肉芽组织过度增生的创面或深部腔隙。鉴于聚乙烯醇泡沫敷料抗拉强度较高、孔隙更密集,因此也需要更高的压力设置。聚氨酯材料的压力值为-16.625 kPa(-125 mmHg)左右,而聚乙烯醇的泡沫需要更高的压力才可将渗出液排除,否则残留的渗出液会阻碍愈合,渗出液中的炎症因子也会损害细胞的生长和增殖。在敷料覆盖下,空气中的细菌和污物无法附着,降低患者在复杂病房环境中病原菌交叉感染的可能性,并且创面内组织液和渗出液中的水分不会过度蒸发,敷料材质的特殊性可使骨与软组织感染的创面维持湿润状态,这有利于肉芽组织的形成。同时NPWT为创伤部位提供向心力,促进创伤边缘向中央聚拢,有助于促进皮缘的延长和愈合[6]。NPWT相较于传统敷料,更换次数少,医护人员负担轻,例如肥胖患者或特殊创面部位,如腘窝、臀部或腹股沟,可有效降低频繁更换辅料及暴露创面导致交叉感染的概率和多重细菌感染的发生。虽然NPWT泡沫敷料因更换次数明显少于传统敷料而广泛应用于临床实践,但较传统敷料仍具有一定局限。泡沫敷料上的微孔允许肉芽组织向内生长,因此,更换时容易破坏创面并引起疼痛,而纱布敷料则因致密的线条和螺旋设计减少了这些问题的发生。另外,随着泡沫在创面置留时间延长,也可能会发生分解并被细菌定植。NUUTILA等[7]为缓解上述敷料所附带的负面影响,研发了一种新型非透水单层成分膜敷料,形成了不需要泡沫敷料即可发挥作用的NPWT技术。 2.2 NPWT减轻伤口水肿 NPWT减轻组织水肿主要是通过:①负压吸引组织间液;②促进淋巴管的生成。文献报道过度的组织肿胀会影响创面愈合,而高达90%的间质液由淋巴循环排除,而非静脉循环系统[8]。创面愈合延迟的原因之一是淋巴再生减少、引流功能障碍和维持液体稳态的代偿机制受损,这导致持续的组织水肿和炎性因子循环不良[9]。组织内的淋巴网络可有效地将富含蛋白质的液体从组织间隙中排出、维持液体平衡并限制水肿的发生。有研究通过小鼠模型证实NPWT具有刺激淋巴网络生成的潜力,NPWT作用于小鼠切口可产生微变形剪切力,引起淋巴管内皮受体1在转录水平上表达上调,淋巴管密度和直径增加,改善淋巴网络的引流,并进一步提高创面愈合的速度。淋巴管内皮受体1是淋巴管上发现的一种白细胞对接受体,可介导巨噬细胞、T淋巴细胞和树突状细胞的运输[10]。创面组织水肿不仅增加细胞间的距离,影响组织细胞间的物质交换,而且减少创面的血液供应,最终使创面有害物质堆积而影响骨与软组织感染的愈合。研究表明,NPWT可直接刺激细胞和拉伸局部组织来增加压力,同时在组织间隙和辅助材料界面之间提供压力梯度,加快细胞外液的排除。BANWELL[11]报道NPWT可以去除创面组织液,减轻组织水肿。在兔耳背侧全层皮肤缺损的急性创面模型中,NPWT可降低创面组织含水量。血管内皮生长因子C是血小板源性生长因子/血管内皮生长因子家族的成员,参与血管、淋巴管生成及内皮细胞的增殖分化并影响血管通透性。血管内皮生长因子C表达的程度与组织水肿程度直接相关[12-13]。淋巴漏动物模型中NPWT组血管内皮生长因子C表达水平在整个实验过程中明显低于对照组,这表明NPWT可以在一定程度上减轻淋巴漏动物模型的真皮水肿[12]。 2.3 NPWT降低细菌载荷 细菌的数量和种类是影响骨与软组织感染治疗效果的重要因素之一,无论是外科清创还是抗生素药物治疗,降低骨与软组织感染创面内细菌载荷均不理想,这是由于清创无法彻底及细菌生物膜的存在。如果将感染组织清创范围扩大到正常组织5 mm内则可将细菌彻底清除,但是临床中对于感染和正常组织辨识困难且部分部位无法彻底清创,导致手术效果大打折扣;细菌生物膜形成是目前抗生素保守治疗失败的重要原因[14-15],细菌生物膜是由细菌嵌入其自身产生的细胞外基质而形成的多细胞结构,由细菌、自身产生的细胞外基质以及表面液体或液-气界面这3种基本成分构成,其水分含量可达97%[16]。细菌生物膜并不是附着在生物或物体表面简单的细胞组合,而是在结构和功能上一个动态变化的生物系统[17]。NPWT在治疗感染方面具有先天优势,原因在于能够不间断地依赖负压吸引组织渗出液及细胞外基质,同时能够将较大的团块、性质柔软的残渣和分泌物分割成颗粒状吸引排出,消除细菌的附着点。NPWT解决了引流管易堵塞的难题,可以及时排出引流组织中的脓性积液、少量坏死组织、异常聚集的各种液态物质[18-20];NPWT压力均匀分布在聚乙烯醇表面,形成全方位的引流,并且聚乙烯醇具有良好的通透性,可允许液体和小颗粒通过[21],因此,NPWT可以通过负压将细菌生物膜中的水介质充分引流,破坏细菌的生长环境,导致生物膜中细菌引流到体外,同时消灭死腔[18],另外聚乙烯醇包裹引流管,周围组织和器官无法直接接触引流管,可防止周围组织被吸入造成缺血、坏死和局部出血等并发症[20]。EIHAGE等[22]发现NPWT通过抑制核因子κB通路破坏糖尿病足创面细菌生长和繁殖的环境,进而降低内毒素和炎症反应。总之,NPWT通过有效排出坏死组织及渗出液、破坏细菌附着面、干扰细菌生物膜环境等方式,可有效降低骨与软组织感染创面的细菌载荷,促进感染病灶的愈合。 2.4 NPWT刺激肉芽组织 健康的肉芽组织和细胞增殖是创面修复的重要阶段。肉芽组织的组成包括成纤维细胞、角质形成细胞、内皮细胞、新生薄壁毛细血管以及细胞外基质的炎症细胞浸润,具有一定的收缩功能。肉芽组织的形成是复杂的,是创面各细胞类型之间相互作用的结果。研究发现,不仅局部微化学环境能够促进肉芽生长,而且机械牵引力也是其生长不可缺少的影响因素。NPWT的内置泡沫和负压的结合可致细胞发生机械微形变,进而刺激肉芽组织生长。SAHIN等[23]在利用NPWT对比干湿敷料治疗压疮中通过三维创面测量仪发现,NPWT和湿转干敷料均可有效治疗压疮,但是NPTW实验组中肉芽组织的形成和创面收缩效果明显优于干湿辅料对照组,证明NPWT是一种压疮治疗的有效方法。NPWT所产生的机械牵引力具有促进肉芽组织生长的作用,通过调节细胞内信号调控蛋白质生成,来促进肉芽组织生成。肉芽组织中的角质形成细胞是皮肤表皮的主要构成成分,HSIAO等[24]通过动物模型实验证明在NPWT干预下表达Ki67的角质形成细胞数量急剧增加,并且细胞外基质也相应增加。 细胞增殖不仅生长因子参与,细胞外机械牵引力也参入其中,使细胞变形、诱导增殖,如果细胞没有变形能力,细胞的增殖能力将明显减弱[25]。文献报道为使细胞对可溶性有丝分裂因子发生反应并增殖,必须在细胞外部施加作用力,使细胞伸展变形,从而诱导细胞增殖的目的;而无受力伸长的细胞则呈球状,生长停滞,出现凋亡的倾向[26]。机械牵引力促进细胞增殖在治疗不同疾病中应用,软组织重建手术中使用组织扩张方式来扩张软组织包膜;颌面外科领域中使用牵引成骨术来延长骨骼[26];同理NPWT干预过程中,细胞受到机械力的影响诱导增殖。这是因为NPWT的机械牵引力通过细胞外基质传递给单个细胞[27],使细胞变形,从而促进细胞增殖。MORYKWAS等[28-29]报道负压吸引实验组创面肉芽组织量比对照组多63.3%,同时也有学者报道负压可促进生长因子及金属基质酶的表达[30-33]。 2.5 NPWT增强血流灌注 组织灌注是创面愈合的必备条件之一,任何创面的愈合都需要血液转运养分和排出代谢废物,骨与软组织感染的创面也是如此,而NPWT可以在一定程度上增加局部组织血流灌注。BOTA等[34]通过分光光度法测量发现负压作用于健康组织后,毛细血管的静脉氧饱和度和血红蛋白浓度会高于基线水平,这表明负压增加组织灌注,可用于高危患者术前预处理或慢性创面的辅助治疗。SEO等[35]发现,NPWT促进了血管内皮生长因子的全体性降低,但增加了循环内皮祖细胞的数量,这表明内皮祖细胞动员是调节缺氧诱导因子1α/血管内皮生长因子通路与血管新生之间的桥梁机制。文献报道通过激光多普勒血流仪检测,在16.625 kPa的负压下家猪创伤模型的血流量增加了4倍[28]。血管张力和血管活性因子是介导真皮微血管形成的主要因素,NPWT作用于细胞外基质的机械力可间接干预血管张力变化进而影响微血管的形成,因此NPWT所产生的机械力在一定程度上可促进微血管的形成[36]。 微血管形成是组织修复中最重要的过程,是由现有的毛细血管或后毛细血管发育而形成的。功能性新生微血管可为创面愈合过程提供稳定的氧和营养物质,对急、慢性创面愈合非常重要。MORYKWAS等[28] 研究关于负压对新鲜皮肤缺损创面血流量的影响,发现负压作用下创面血流量较多,其峰值可达基线水平血流量的4倍之多,其作用机制是创面局部与周围组织表面的压力差可促进创面血流灌注。血管生成受多种创面微环境的影响,包括缺氧和各种趋化因子:①缺氧是创面微环境的重要组成部分,是启动血管生成的主要刺激因素之一;创面形成后,由于局部血液循环紊乱,创面边缘细胞耗氧量增加,创面形成局部缺氧微环境。②缺氧对调节缺氧诱导因子1α起重要作用并刺激血管内皮生长因子的表达。血管内皮生长因子是重要的血管新生因子,也参与胶原沉积和创面上皮细胞的形成[37-38]。目前研究表明NPWT可以促进缺氧梯度的增宽,提高血管内皮生长因子表达,促进生理功能正常的微血管形成,而传统的封闭敷料主要产生蜿蜒脆弱的无效血管[37]。在NPWT相关研究中,血管内皮生长因子是研究较多的生长因子之一[39]。NPWT的物理作用不仅可以调节血管内皮生长因子及其受体的基因表达,还可通过现有血管的“非血管生成扩张”来影响新血管的形成。KILARSKI等[40]发现在创面收缩期间活化的成纤维细胞或肌成纤维细胞可产生一定的组织张力,介导血管系统的转位并定向。机械力使新生血管从现有的血管床延伸,形成功能性的血管环,肉芽组织伴随着血管环的延伸而生长。血管内皮生长因子受体2的阻断实验证实,在排除血管内皮生长因子作用的微环境条件下,生物力学可介导新生血管的生长。NPWT促进微血管形成的次要机制包括创面液体的持续引流,这有助于调节创面炎症微环境,有利于新血管的形成。创面愈合过程可根据微血管的生成状态分为早期和晚期。研究发现NPWT在创面愈合早期通过提高血管生成素2表达水平,降低血管紧张素1表达水平,刺激微血管失稳和退化,进而促进血管生成;在创面愈合后期,NPWT则通过提高血管紧张素1的表达水平以及酪氨酸激酶受体2的磷酸化水平促进创面微血管成熟和稳定[41]。 2.6 NPWT对周围神经的影响 骨与软组织感染病灶的愈合过程不仅需要炎症细胞的参与,周围神经系统的参与也具有重要作用。目前已有文献证明外周神经末梢系统在皮肤稳态及其病灶愈合过程中起到关键作用[42-43],外周神经末梢所释放的感觉神经肽物质P[44]、降钙素基因相关肽和神经生长因子等神经肽可以有效促进慢性溃疡的愈合[45-46]。皮肤中的部分神经纤维终止于真皮层和表皮的游离神经末梢,在某种刺激下能够释放感觉神经肽物质P和降钙素基因相关肽。神经生长因子是由创面周边固有细胞所合成分泌,当受到外界因素干预时,被释放至周围神经末梢的突触间隙中,然后刺激神经肽的释放。在创面愈合的炎症反应期,白细胞在感觉神经肽物质P趋化作用下从扩张的毛细血管中扩散外渗至创面进行炎症反应[47],同时巨噬细胞吞噬外来物质,刺激施万细胞和成纤维细胞增殖并分泌神经生长因子,进一步刺激感觉神经肽物质P的产生。感觉神经肽物质P和降钙素基因相关肽对皮肤组织内的血管具有扩张作用。在去神经化的组织中,由于去神经化所诱发的血管平滑肌脱敏效应,微血管反应较正常生理状态明显减弱。 目前主要存在2种有关外界机械力干预激活周围神经末梢释放神经肽的假设:①部分学者认为外界机械力通过皮肤直接将作用力传递至周围神经末梢的机械敏感痛觉感受器,刺激神经发育,同时增加感觉神经肽物质P和降钙素基因相关肽的表达和释放[48];②多数学者支持皮下组织内的固有细胞表面有机械感受器,在外界机械力刺激的干预下,合成并释放神经生长因子,作用于神经末梢,进而促进神经末梢释放感觉神经肽物质P和降钙素基因相关肽。YOUNAN等[49]在小鼠背侧表皮施加特定张力的模型中发现,皮肤拉伸导致感觉神经肽物质P、降钙素基因相关肽和神经生长因子的表达增加。当皮肤被施加负压时,负压产生的机械力作用于创面组织使其发生微变形而分散机械力。固有细胞表面存有敏感的机械感受器,在外界机械力干预下,合成并分泌神经生长因子至突触间隙,周围神经末梢通过摄取神经生长因子来局部刺激感觉神经肽物质P和降钙素基因相关肽从神经末梢释放。适当的神经支配和正常神经肽水平对于创面愈合至关重要,NPWT可用于去神经化疾病如糖尿病足、截瘫患者压疮等慢性延迟愈合创面的治疗。 2.7 NPWT调节机体免疫 在对抗骨与软组织感染的病情进展中,通过患者自身的生物免疫功能使患者痊愈,无疑是最理想的治疗方法。抗生素治疗实际上是在辅助生物免疫的前提下对抗细菌侵害,而生物免疫仍是主导地位。NPWT通过机械外力参与到感染部位的局部免疫微环境来促进骨与软组织感染的愈合。免疫微环境由免疫细胞和免疫因子共同构成,对骨与软组织感染发病机制的理解具有重要意义。文献报道活性氧与创面愈合密切相关,直接参与创面愈合过程的多个阶段[50]。QIU等[51]研究发现NPWT可通过影响氧化应激来促进创面修复。氧化应激是指身体内部亲氧化剂和抗氧化剂的不平衡。低浓度的活性氧参与细胞内多种信号转导通路的调节,并为吞噬细胞提供能量,而高浓度的活性氧可直接与细胞脂质、蛋白质和DNA反应,造成细胞损伤和死亡如脂质过氧化。平衡活性氧的正负作用,减少氧化应激造成的损伤对创面愈合至关重要。在NPWT干预下,丙二醛水平在第3,5,7天显著降低;超氧化物歧化酶和过氧化氢酶的表达在第3,5天显著降低,其说明NPWT一定程度上能够通过影响氧化应激进而促进创面的修复。汪涛等[52]利用NPWT治疗糖尿病足溃疡的临床试验发现NPWT可降低肿瘤坏死因子α、白细胞介素6含量,降低环氧化酶和一氧化氮化酶蛋白水平,抑制糖尿病足创伤的局部炎症反应,促进创面愈合。程海霞等[53]在骨折创面感染研究中发现NPWT组患者治疗后超敏C-反应蛋白、白细胞介素6、白细胞介素8、白细胞介素10、血沉水平较对照组下降明显,这表明NPWT有利于降低炎症因子水平,减轻炎症反应,感染创面愈合。Piezo1是细胞感应外界机械力的一种感受器,Piezo1特异性促进胞外钙离子内流,实现外环境机械力到胞内生物学信号的转换和传递[54]。Piezo1鉴定工作获得了2021年度诺贝尔生理与医学奖。包括静压(Hydrostatic Pressure)、流体剪切应力(Laminar Flow Forces)和膜拉伸(Cellular Stretch)在内的机械力(Mechanical Forces)会激活Piezo1通道开放,从而允许阳离子,尤其是Ca2+流入细胞。免疫细胞可以通过机械敏感受体来感知这些变化,进而表现出不同的免疫应答反应[55]。最近,厦门大学周大旺教授团队研究发现,巨噬细胞中Piezo1可以通过与Toll样受体信号通路相互作用来直接调控巨噬细胞对于病原体的清除[56]。加州大学Wendy F. Liu团队也发现Piezo1调控巨噬细胞炎症反应、吞噬作用[57]。Piezo1分子是巨噬细胞中唯一高丰度表达的机械力感受器分子[58]。负压干预可以影响巨噬细胞的极化,这与负压作用下产生的机械牵引力引起细胞变形、改变细胞骨架有关,从而影响细胞的功能状态。同时,负压可激活Rho-Rho-Kinase Pathway、ERK/MAPK Pathway、Cyclooxygenase Pathway及离子接受通道[59-61],促进细胞因子、炎症递质等的释放,从而改变细胞功能,细胞功能的改变反过来促进相关因子的释放这样形成相互作用的循环系统。 2.8 NPWT对骨组织的影响 骨与软组织感染的发生大都伴有骨和软组织缺损,与软组织缺损相比,骨组织缺损对机体功能的影响更大,如腰椎部位感染侵犯椎体时,相邻椎体出现失稳,引起脊柱侧弯压迫神经等严重并发症。因此,对于骨组织缺损,骨科医生需要更加重视。骨移植目前是比较主流的解决方案,但提高了供体部位疼痛、发病率、并发症以及病理骨折和大面积骨缺损的风险[62]。而骨组织工程的兴起为骨缺损治疗提供了一种崭新的方案,骨组织具有强大的再生恢复能力。人骨髓间充质干细胞在骨再生过程中起重要作用,这来源于可对机械刺激产生反应的骨前体细胞[63]。文献证明,在持续负压下,NPWT可通过增强间充质干细胞增殖、成骨细胞分化、血管内皮生长因子、骨形态发生蛋白2和骨桥蛋白的表达来加速骨再生[64]。牵张成骨是口腔颌面外科最常用的组织工程相关骨增强技术之一。在牵张成骨中活跃的骨形成是由于增强骨形成标记物如骨形态发生蛋白2、骨形态发生蛋白4、降钙素、Ⅰ型胶原在机械作用力下高表达,这表明机械外力在骨的增殖与分化中起到重要作用。研究证实,人骨膜源性干细胞也同样可以看作是活跃的骨膜源性成骨细胞,低频低强度的周期性张力对人骨膜源性干细胞的体外成骨分化有积极影响[65]。NWPT产生的微机械力为骨祖细胞的增殖和分化提供了适宜的外部条件,从而加速骨缺损修复及生物体骨生理功能的恢复。"

| [1] ARGENTA LC, MORYKWAS MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997; 38(6):563-576; discussion 577. [2] TORBRAND C, ANESATER E, BORGQUIST O, et al. Mechanical effects of negative pressure wound therapy on abdominal wounds - effects of different pressures and wound fillers. Int Wound J. 2018;15(1):24-28. [3] LIU Z, DUMVILLE JC, HINCHLIFFE RJ, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. 2018;10(10):CD010318. [4] WANG J, YANG Y, XING W, et al. Safety and efficacy of negative pressure wound therapy in treating deep surgical site infection after lumbar surgery. Int Orthop. 2022;46(11):2629-2635. [5] HU FX, HU XX, YANG XL, et al. Treatment of large avulsion injury in perianal, sacral, and perineal regions by island flaps or skin graft combined with vacuum assisted closure. BMC Surg. 2019;19(1):65. [6] LIVINGSTONE JP, SINGH D, MURRAY PC. An In Vitro Study Measuring the Effects of Circumferential and Near-Circumferential Closed Incisional Negative Pressure Wound Therapy Dressings. Cureus. 2021;13(4):e14389. [7] NUUTILA K, YANG L, BROOMHEAD M, et al. Novel negative pressure wound therapy device without foam or gauze is effective at -50 mmHg. Wound Repair Regen. 2019;27(2):162-169. [8] MELIN MM. Understanding wound care from a venous and lymphatic perspective. J Wound Care. 2020;29(Sup9):S6-S7. [9] YOSHIDA S, KOSHIMA I, HAMADA Y, et al. Lymphovenous Anastomosis Aids Wound Healing in Lymphedema: Relationship Between Lymphedema and Delayed Wound Healing from a View of Immune Mechanisms. Adv Wound Care (New Rochelle). 2019;8(6):263-269. [10] RANDOLPH GJ, IVANOV S, ZINSELMEYER BH, et al. The Lymphatic System: Integral Roles in Immunity. Annu Rev Immunol. 2017;35:31-52. [11] BANWELL PE. Topical negative pressure therapy in wound care. J Wound Care. 1999;8(2):79-84. [12] YUAN Y, NIU Y, XIAO W, et al. The Effect and Mechanism of Negative Pressure Wound Therapy on Lymphatic Leakage in Rabbits. J Surg Res. 2019;235: 329-339. [13] IWASAKI D, YAMAMOTO Y, MURAO N, et al. Establishment of an Acquired Lymphedema Model in the Mouse Hindlimb: Technical Refinement and Molecular Characteristics. Plast Reconstr Surg. 2017;139(1):67e-78e. [14] LIANHUA Y, YUNCHAO H, GUANGQIANG Z, et al. The effect of iatrogenic Staphylococcus epidermidis intercellar adhesion operon on the formation of bacterial biofilm on polyvinyl chloride surfaces. Surg Infect (Larchmt). 2014; 15(6):768-773. [15] CHEN L, WEN YM. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011;3(2):66-73. [16] DONLAN RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002; 8(9):881-890. [17] HALL-STOODLEY L, COSTERTON JW, STOODLEY P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004; 2(2):95-108. [18] LI W, JI L, TAO W. Effect of vacuum sealing drainage in osteofascial compartment syndrome. Int J Clin Exp Med. 2015;8(9):16112-16116. [19] QU J, YAN R, WANG L, et al. Free dermatoplasty combined with vacuum sealing drainage for the treatment of large-area soft tissue defects accompanied by bone exposure in the lower leg. Exp Ther Med. 2013;5(5): 1375-1380. [20] FLEISCHMANN W, STRECKER W, BOMBELLI M, et al. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg. 1993; 96(9):488-492. [21] LIU X, LIANG J, ZAO J, et al. Vacuum Sealing Drainage Treatment Combined with Antibiotic-Impregnated Bone Cement for Treatment of Soft Tissue Defects and Infection. Med Sci Monit. 2016;22:1959-1965. [22] ELHAGE KG, AWAD ME, IRFAN FB, et al. Closed-incision negative pressure therapy at -125 mmHg significantly reduces surgical site complications following total hip and knee arthroplasties: A stratified meta-analysis of randomized controlled trials. Health Sci Rep. 2022;5(1):e425. [23] SAHIN E, RIZALAR S, OZKER E. Effectiveness of negative-pressure wound therapy compared to wet-dry dressing in pressure injuries. J Tissue Viability. 2022;31(1):164-172. [24] HSIAO HY, HSIEH WC, CHANG FC, et al. The Effect of Negative Pressure on Wound Healing and Regeneration in Closed Incisions under High Tension: Evidence from Animal Studies and Clinical Experience. J Clin Med. 2022; 12(1):106. [25] HUANG S, INGBER DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1(5):E131-E138. [26] SAXENA V, HWANG CW, HUANG S, et al. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114(5):1086-1096; discussion 1097-1088. [27] HUANG C, LEAVITT T, BAYER LR, et al. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301-331. [28] MORYKWAS MJ, ARGENTA LC, SHELTON-BROWN EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553-562. [29] MORYKWAS MJ, FALER BJ, PEARCE DJ, et al. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg. 2001;47(5):547-551. [30] TRENGOVE NJ, BIELEFELDT-OHMANN H, STACEY MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8(1):13-25. [31] MURPHY MA, JOYCE WP, CONDRON C, et al. A reduction in serum cytokine levels parallels healing of venous ulcers in patients undergoing compression therapy. Eur J Vasc Endovasc Surg. 2002;23(4):349-352. [32] DASU MR, BARROW RE, SPIES M, et al. Matrix metalloproteinase expression in cytokine stimulated human dermal fibroblasts. Burns. 2003;29(6):527-531. [33] VAALAMO M, LEIVO T, SAARIALHO-KERE U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol. 1999;30(7):795-802. [34] BOTA O, MARTIN J, HAMMER A, et al. Topical negative pressure wound therapy enhances the local tissue perfusion - A pilot study. Microvasc Res. 2022;140:104301. [35] SEO SG, YEO JH, KIM JH, et al. Negative-pressure wound therapy induces endothelial progenitor cell mobilization in diabetic patients with foot infection or skin defects. Exp Mol Med. 2013;45(11):e62. [36] BANWELL P, TEOT L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Tissue Viability. 2006;16(1):16-24. [37] ERBA P, OGAWA R, ACKERMANN M, et al. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg. 2011;253(2):402-409. [38] GREENBERG JI, SHIELDS DJ, BARILLAS SG, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008; 456(7223):809-813. [39] GLASS GE, MURPHY GF, ESMAEILI A, et al. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg. 2014; 101(13):1627-1636. [40] KILARSKI WW, SAMOLOV B, PETERSSON L, et al. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med. 2009;15(6): 657-664. [41] MA Z, SHOU K, LI Z, et al. Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp Ther Med. 2016;11(4):1307-1317. [42] BARKER AR, ROSSON GD, DELLON AL. Wound healing in denervated tissue. Ann Plast Surg. 2006;57(3):339-342. [43] ROOSTERMAN D, GOERGE T, SCHNEIDER SW, et al. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006; 86(4):1309-1379. [44] DELGADO AV, MCMANUS AT, CHAMBERS JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med (Maywood). 2005;230(4):271-280. [45] TODA M, SUZUKI T, HOSONO K, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62(6):352-359. [46] NICO B, MANGIERI D, BENAGIANO V, et al. Nerve growth factor as an angiogenic factor. Microvasc Res. 2008;75(2):135-141. [47] RICHARDS AM, FLOYD DC, TERENGHI G, et al. Cellular changes in denervated tissue during wound healing in a rat model. Br J Dermatol. 1999; 140(6):1093-1099. [48] SHUN CT, CHANG YC, WU HP, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004; 127(Pt 7):1593-1605. [49] YOUNAN G, OGAWA R, RAMIREZ M, et al. Analysis of nerve and neuropeptide patterns in vacuum-assisted closure-treated diabetic murine wounds. Plast Reconstr Surg. 2010;126(1):87-96. [50] YIP WL. Influence of oxygen on wound healing. Int Wound J. 2015;12(6): 620-624. [51] QIU X, WU Y, ZHANG D, et al. Roles of Oxidative Stress and Raftlin in Wound Healing Under Negative-Pressure Wound Therapy. Clin Cosmet Investig Dermatol. 2021;14:1745-1753. [52] 汪涛, 赵珺, 于敏, 等. 负压吸引疗法通过减轻炎症反应促进糖尿病足溃疡愈合[J]. 上海交通大学学报(医学版),2016,36(8):1159-1164. [53] 程海霞, 吴松梅, 明晓锋, 等. VSD对骨折创面感染患者血清炎症因子及骨性标志物的影响[J]. 中华医院感染学杂志,2019,29(23):3598-3602. [54] SOLIS AG, BIELECKI P, STEACH HR, et al. Author Correction: Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;575(7784):E7. [55] MA S, DUBIN AE, ZHANG Y, et al. A role of PIEZO1 in iron metabolism in mice and humans. Cell. 2021;184(4):969-982.e913. [56] GENG J, SHI Y, ZHANG J, et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat Commun. 2021;12(1):3519. [57] ATCHA H, JAIRAMAN A, HOLT JR, et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat Commun. 2021;12(1):3256. [58] WANG Y, YANG H, JIA A, et al. Dendritic cell Piezo1 directs the differentiation of T(H)1 and T(reg) cells in cancer. Elife. 2022;11:e79957. [59] ILHAN E, BUYUKAFSAR K, TIFTIK RN, et al. Effects of the Rho/Rho-Kinase Pathway on Perfusion Pressure in the Isolated-Perfused Rat Hind Limb Vascular Bed. Balkan Med J. 2021;38(5):304-309. [60] AN C, HUANG Y, LI M, et al. Vesicular formation regulated by ERK/MAPK pathway mediates human erythroblast enucleation. Blood Adv. 2021;5(22): 4648-4661. [61] KERINGER P, FUREDI N, GASZNER B, et al. The hyperthermic effect of central cholecystokinin is mediated by the cyclooxygenase-2 pathway. Am J Physiol Endocrinol Metab. 2022;322(1):E10-E23. [62] WANG R, THAYER P, GOLDSTEIN A, et al. Interaction of material stiffness and negative pressure to enhance differentiation of bone marrow-derived stem cells and osteoblast proliferation. J Tissue Eng Regen Med. 2020;14(2):295-305. [63] FILIPOWSKA J, REILLY GC, OSYCZKA AM. A single short session of media perfusion induces osteogenesis in hBMSCs cultured in porous scaffolds, dependent on cell differentiation stage. Biotechnol Bioeng. 2016;113(8): 1814-1824. [64] ZHU J, WANG F, YAN L, et al. Negative pressure wound therapy enhances bone regeneration compared with conventional therapy in a rabbit radius gap-healing model. Exp Ther Med. 2021;21(5):474. [65] LEE JM, KIM MG, BYUN JH, et al. The effect of biomechanical stimulation on osteoblast differentiation of human jaw periosteum-derived stem cells. Maxillofac Plast Reconstr Surg. 2017;39(1):7. |
| [1] | Chen Zan, Lei Fei, Ye Fei, Zhou Qingzhong, Yuan Hao, Zheng Lipeng, Zha Xian, Feng Daxiong. Relationship between drainage time and early efficacy after short-segment lumbar fusion [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 927-933. |
| [2] | Xu Jing, Lyu Huixin, Bao Xin, Zhang Yi, Wang Yihan, Zhou Yanmin. Application of near infrared responsive hydrogels in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 486-492. |
| [3] | Xu Xingxing, Wen Chaoju, Meng Maohua, Wang Qinying, Chen Jingqiao, Dong Qiang. Carbon nanomaterials in oral implant [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1062-1070. |
| [4] | Xu Qijing, Yang Yichun, Lei Wei, Yang Ying, Yu Jiang, Xia Tingting, Zhang Meng, Zhang Tao, Zhang Qian. Advances and problems in cell-free treatment of diabetic skin chronic wounds [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 962-969. |
| [5] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [6] | Li Li, Li Xiao, Li Duchenhui, Zhang Jie, Xiao Tianjiao, Kang Jiabing, Tian Ai. Regulation of interleukin-4 on osteoclast differentiation during bone regeneration guided by bone replacement materials [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5455-5461. |
| [7] | Leng Siyi, Pu Rui, Chen Ziyang, Yang Qihang, Song Yongjing, Liu Hui. Roles of exercise intervention in intestinal flora in autoimmune diseases [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5219-5226. |
| [8] | Fan Haomei, Xiao Dongqin, Shi Feng, Luo Xuwei, Wei Jianlin, Zhuang Huadi, Liu Jinhui, Zhao Juhua. Preparation of calcium silicate microspheres loaded with epigallocatechin gallate and investigation on its antibacterial performance [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4769-4775. |
| [9] | Li Yaohua, Zeng Fengjiao, Chen Bin, Fan Qin, Bai Guohui. Research advances in drug coatings for prevention of peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4912-4920. |
| [10] | Zhou Jie, Pei Xibo, Wan Qianbing. Advances and biological application of asymmetric dressings [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 434-440. |
| [11] | You Aijia, Li Wenjie, Zhou Junli, Li Chun. Systematic evaluation of six dressings on wound safety following total hip and knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 486-492. |
| [12] | Liu Zhilun, Guan Zhiyu, Jiang Taiping, Li Chengxi, Liu Zhaoming. Mechanism of stone balm on the healing of infected refractory wounds in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(26): 4126-4131. |
| [13] | Pan Wei, Li Shuyang, Liu Jinhui, Liang Cheng, Duan Ke, Chen Xingtao. Preparation and performance evaluation of calcium sodium carboxymethyl cellulose/ hydroxypropyltrimethyl ammonium chloride chitosan multilayer dressing [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3949-3955. |
| [14] | Shi Guangbo, Li Xiuting, Liu Xingcui, Guo Xianhu, Li Longfei, Wang Wenqing. Ability of medical wound dressings to resist penetration of bloodborne pathogens [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3937-3941. |
| [15] | Liu Jichao, Zhao Jinlong, Yu Yang. Silk fibroin collagen composite scaffold combined with platelet-rich plasma for repairing skin injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 3971-3976. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||