Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (25): 4085-4092.doi: 10.12307/2023.541
Previous Articles Next Articles
Role and regulation of macrophages in biomaterial-mediated fibrosis formation
Deng Moyuan2, 3, Peng Kun1
- 1Chongqing Medical and Pharmaceutical College, Chongqing 401331, China; 2National & Regional United Engineering Lab of Tissue Engineering, Department of Orthopaedics, Southwest Hospital, the Third Military Medical University, Chongqing 400038, China; 3Chongqing Forwos Medical Equipment Co., Ltd., Chongqing 400714, China
-
Received:2022-08-19Accepted:2022-09-20Online:2023-09-08Published:2023-01-18 -
Contact:Peng Kun, MD, Associate professor, Chongqing Medical and Pharmaceutical College, Chongqing 401331, China -
About author:Deng Moyuan, PhD, Assistant researcher, National & Regional United Engineering Lab of Tissue Engineering, Department of Orthopaedics, Southwest Hospital, the Third Military Medical University, Chongqing 400038, China; Chongqing Forwos Medical Equipment Co., Ltd., Chongqing 400714, China -
Supported by:The National Natural Science Foundation of China, No. 81771995 (to DMY); The Natural Science Foundation of Chongqing, No. cstc2018jcyjAX0828 (to PK)
CLC Number:
Cite this article
Deng Moyuan, Peng Kun. Role and regulation of macrophages in biomaterial-mediated fibrosis formation[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(25): 4085-4092.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
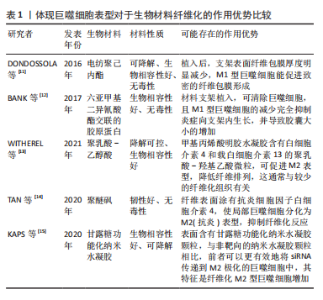
2.1 巨噬细胞在生物材料纤维化形成中的作用及相关机制 2.1.1 生物材料介导纤维化的形成 生物材料启动的损伤修复反应通常始于血管化组织的损伤。从受损血管释放的血浆蛋白吸附在材料表面并形成临时细胞外基质,为材料表面提供了一个可招募宿主细胞、储存细胞、可持续释放各种细胞因子、生长因子及活性蛋白的微环境,称为血栓或伤口凝块[3]。血栓形成意味着局部组织进入急性炎症阶段[2],在最初发病的几个小时内,中性粒细胞、脱粒肥大细胞以及单核细胞相继招募到生物材料表面[4];而后单核细胞分化成为巨噬细胞,最终成为植入物表面及周围组织的主要细胞群,比例可达到骨髓细胞总数的1/6[5]。 巨噬细胞在体内存在的数天内,可吞噬死细胞、受损组织以及植入物可能的降解产物,分化为肌成纤维细胞[6],也会与其他细胞,如角质形成细胞、内皮细胞和脂肪细胞在多种细胞因子作用下招募和激活损伤组织附近的成纤维细胞[7]。进一步地,募集到的成纤维细胞会经历如下几步流程:①与材料表面吸附的临时细胞外基质形成黏着斑;②黏着斑激活α-平滑肌肌动蛋白;α-平滑肌肌动蛋白应力纤维,并产生高收缩力;③细胞收缩使临时细胞外基质转变成硬性细胞外基质,并激活诸如整合素αvβ1;④活化的整合素αvβ1结合细胞外基质中转化生长因子β1潜伏相关肽;⑤细胞收缩力打开潜伏相关肽并释放活性转化生长因子β1;⑥活性转化生长因子β1与其受体复合物结合,转化生长因子β1/信号转导分子3的信号途径驱动促纤维化程序,进一步激活成纤维细胞为肌成纤维细胞、使硬性的胞外基质纤维组织包裹植入的生物材料[8-9]。 2.1.2 不同表型巨噬细胞在生物材料纤维化中的作用 巨噬细胞是高度可塑性的细胞,受微环境影响转化为多种表型,其中以辅助 T 细胞命名法通常归为经典激活/促炎性“M1”型巨噬细胞,以及替代激活/抗炎性“M2”型巨噬细胞[10]。其中,在组织修复的背景下,由脂多糖、干扰素γ或肿瘤坏死因子α等促炎信号刺激巨噬细胞为M1型;白细胞介素4或白细胞介素13刺激巨噬细胞为M2a型[1]。在这篇综述中,使用M1/M2命名法,并探讨不同表型巨噬细胞在骨修复材料纤维化中的作用。 将电纺聚己内酯支架植入C57BL/6-Tg小鼠皮下2-4周后形成致密的纤维包膜,且包膜中标记了CD68+的巨噬细胞多为M1型;然而,将聚己内酯支架植入用氯膦酸盐脂质体制备的巨噬细胞耗竭小鼠皮下4周,支架表面纤维包膜厚度明显减少,同时异物巨细胞的数量也相应减少,提示产生M1型巨噬细胞能促进致密的纤维包膜形成[11]。另有研究则展示了M1型巨噬细胞在纤维化形成中矛盾的作用,用六亚甲基二异氰酸酯交联的胶原蛋白片,并用小分子AP20187制备了巨噬细胞凋亡的C57BL/6小鼠,相比于正常小鼠,植入巨噬细胞凋亡小鼠的上述片表面,M1 型巨噬细胞表达降低,但纤维囊厚度显著增加,提示该支架表面M1型巨噬细胞不能促进材料纤维化[12]。虽然两个实验的结果相悖,但都说明M1型巨噬细胞在生物材料介导纤维化发挥关键的调节作用。 同样,M2型巨噬细胞在纤维化中也发挥重要作用。WITHEREL等[13]研究发现含M2 型巨噬细胞条件培养基可调节成纤维细胞促进纤维基质形成;含白细胞介素4和白细胞介素13的聚乳酸-乙醇酸微粒的甲基丙烯酸明胶水凝胶植入小鼠皮下,释放白细胞介素4和白细胞介素13,促进M2型巨噬细胞形成和减少纤维化组织形成。类似地,TAN等[14]采用涂有白细胞介素4的聚醚砜中空纤维封装膜,促进局部巨噬细胞极化为M型,抑制纤维化反应。相反,也有研究显示,M2型巨噬细胞是促纤维化的巨噬细胞,能促进肿瘤、肝和肾等组织的纤维化,提示不同情况下M2型巨噬细胞在纤维化形成中的不同作用[15],见表1。"

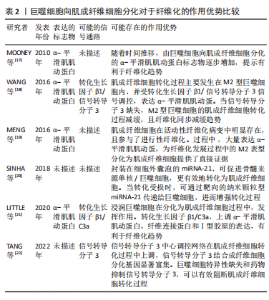
在生物材料表面巨噬细胞融合为多核的异物巨细胞也被认为是一种独特的巨噬细胞表型[1,16]。异物巨细胞不能吞噬超大物体,但促进活性氧物和细胞外基质降解蛋白酶的产生,损坏植入物和周围组织同时,可调节植入物周围细胞因子的浓度,如血小板衍生生长因子的浓度,招募成纤维细胞以促进纤维化形成[2]。 2.1.3 巨噬细胞向肌成纤维细胞分化在生物材料纤维化中的作用 生物材料植入物招募到巨噬细胞,除了融合成异物巨细胞和招募成纤维细胞外,还会分化为肌成纤维细胞。MOONEY等[17]发现鸡蛋白引起的异物反应导致其表面包裹了纤维组织,且纤维组织中标记F4/80+和Ly6C+巨噬细胞从圆形变为梭形,并表达肌成纤维细胞的α-平滑肌肌动蛋白标志物。WANG等[18]研究表明,在肾移植失败的人类患者的纤维化病变活检中,也发现了这一现象,即CD68+、CD206+的M2型巨噬细胞也表达α-平滑肌肌动蛋白,也就是认为M2型巨噬细胞可转化为肌成纤维细胞,并促进纤维化。 MENG等[19]将这一现象表述为巨噬细胞分化为肌成纤维细胞。SINHA等[20]通过一系列敲除模型、免疫荧光染色、转录组分析和体外培养等实验证实,在伤口愈合过程中的肉芽组织有2/3以上的肌成纤维细胞是骨髓来源单核/巨噬细胞。HAIDER等[6]通过LysM(Cre/+)、ROSA26(EYFP/+)转基因小鼠体内追踪,证实肌成纤维细胞参与了心肌梗死的纤维化;并且在体外无细胞因子刺激下的巨噬细胞,在4周后转变为共表达肌成纤维细胞的α-平滑肌肌动蛋白标志物。后续不断有研究报道,肌成纤维细胞参与包括皮肤伤口愈合以及视网膜、肿瘤、心脏等组织纤维细胞的形成[21-22]。BEZHAEVA等[23]在慢性肾病小鼠植入弹性共聚物,组织工程血管所形成纤维包囊中,26%的肌成纤维细胞共表达巨噬细胞标志物 CD68,提示肌成纤维细胞也参与高分子材料介导的纤维化进程。 文章通过分析上述研究成果,可以基本明确两点:一是由巨噬细胞分化而来的肌成纤维细胞,可以引起α-平滑肌肌动蛋白标志物表达,对于纤维化过程有直接作用;二是生物材料存在时,仍然可观察到因巨噬细胞而引起的纤维化。基于此,为了改善生物材料植入后纤维化程度,可否考虑由巨噬细胞向肌成纤维细胞转化涉及的信号调控,可能成为下一阶段的研究方向。巨噬细胞向肌成纤维细胞分化对于纤维化的作用优势见表2。"
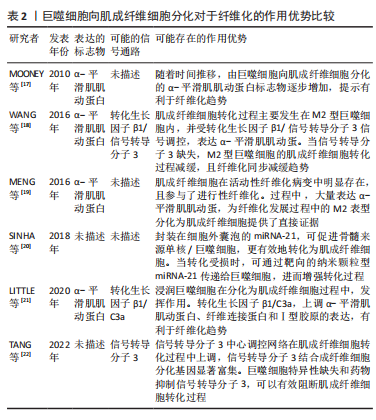
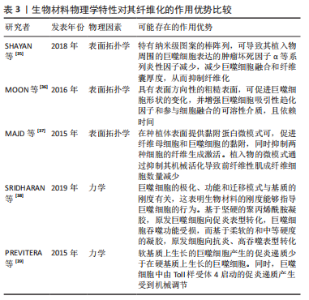
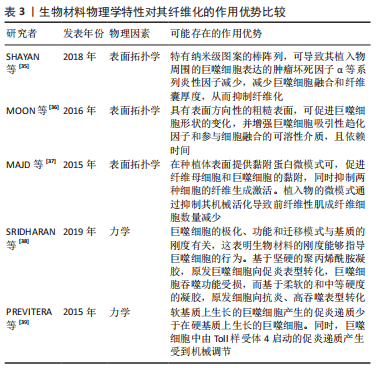
.1.4 基于巨噬细胞的促纤维化过程的相关信号通路 在异物反应中,巨噬细胞引起的炎性信号是纤维化的重要因素。M1 型是促炎巨噬细胞,其通过crosstalk方式激活NLRP3炎症小体途径。M1型巨噬细胞表达的炎性因子白细胞介素6可诱导成纤维细胞的Stat3信号传导并促进纤维化[24]。有研究也报道了M2巨噬细胞促纤维化的相关信号通路。白细胞介素4 和白细胞介素13是常用的M2型巨噬细胞的诱导因子。白细胞介素4与巨噬细胞中的Ⅰ型受体IL-4Rα结合,启动向M2型的极化;而M2a巨噬细胞分泌的Retnla抵抗素样α可直接促进成纤维细胞中胶原蛋白修饰酶的表达,促进胶原纤维的组装,加速了纤维化进程[25]。白细胞介素13则通过与其Ⅱ型受体结合,启动极化及下游促纤维化因子转化生长因子β1的表达[26]。 在巨噬细胞向肌成纤维细胞的分化中,Kruppe样因子、脂质磷酸酶、信号转导分子3等转录因子发挥重要作用[18,20]。Kruppe样因子最近被认为是一种髓系转录因子,它的沉默有利于纤维化的关键事件——上皮-间质转化。同样脂质磷酸酶的沉默也会促进纤维化转变[27-28]。SINHA等[20]发现Kruppe样因子和脂质磷酸酶是miRNA-21的靶标,包裹miRNA-21的细胞外囊泡可促进巨噬细胞向肌成纤维细胞转变[1,29-30]。另一个推进纤维化进程的是信号转导分子3信号途径,该途径同样促进了肾纤维化和心肌组织纤维化的肌成纤维细胞转化进程 [6,18,31]。除了转录因子,A2B腺苷受体也被认为是糖尿病肾纤维化中肌成纤维细胞转化的重要因素,对其进行阻断,可减少巨噬细胞的浸润和肌成纤维细胞形成[32]。 不同表型的巨噬细胞,其纤维化信号通路有可能存在差异。另外,从巨噬细胞促纤维化过程的相关信号通路分析,Stat3、转化生长因子β1、Kruppe样因子、脂质磷酸酶及信号转导分子3等,都有可能促进纤维化。因此,基于上述信号或因子的调控,可能会改善生物材料植入物的纤维化。 2.2 调控巨噬细胞行为以拮抗生物材料纤维化的策略 设计调节巨噬细胞行为的生物材料已成为再生医学和组织工程中一种很有前景的策略。文章前部分,对不同表型巨噬细胞在生物材料纤维化中的作用、巨噬细胞向肌成纤维细胞分化在生物材料纤维化中的作用,以及基于巨噬细胞的促纤维化形成相关信号通路做了综述。接下来,将重点介绍通过改变生物材料物理性能和化学性能,以调控巨噬细胞行为,以期为获得抑制生物材料植入物纤维化的方案,向研究者提供思路和方向。 2.2.1 基于生物材料物理特性的调控策略 生物材料纤维化进程中,巨噬细胞细胞行为和生化信号的改变与材料生物物理特性有关,例如材料拓扑学和材料力学等[33]。虽然巨噬细胞响应生物材料物理特性的机制仍不明,但是现有不少研究聚焦在通过调节材料的物理特性进而来控制巨噬细胞行为,以抑制纤维化形成[34]。 基于材料表面拓扑学的调控策略:研究表明,植入物材料表面粗糙度和微观形貌,可以减少巨噬细胞的炎性因子表达,从而控制纤维化形成[2,35-36]。SHAYAN等[35]研究金属合金玻璃表面 0-200 nm直径纳米棒图案对巨噬细胞融合及纤维化的影响,发现具有粒径约为55 nm图案表面的植入物,可以使巨噬细胞表达的肿瘤坏死因子α等系列炎性因子的表达减少,也表现出巨噬细胞融合和纤维囊厚度减少的现象。MOON等[36]在硅晶圆片表面制备了低/高粗糙度不同形貌的新型凹槽,较之光滑表面,高粗糙度表面更易使巨噬细胞融合形成异物巨细胞,促进纤维化。MAJD等[37]在聚二甲基硅氧烷材料表面制备不同微米间距、不同微米长度的点状纤连蛋白涂层,较之植入大鼠皮下30 d的无涂层圆盘 ,具备4 μm×2 μm 纤连蛋白微点涂层的材料表面巨噬细胞黏附减少、肌成纤维细胞和纤维包膜厚度均明显减少。由此可见,在植入物材料表面通过构建小于100 nm图案表面,或者强化生物材料表面粗糙程度,从而优化植入物的材料表面拓扑结构,可能降低巨噬细胞融合或表达炎性因子,均有可能减少巨噬细胞引起的纤维化。 基于材料力学性能的调控策略:研究显示,生物材料纤维化与材料力学硬度(刚性)有关[33],植入物材料基材的刚性可能引发调控巨噬细胞炎性因子的分泌。SRIDHARAN 等[38]制备了从软基质(11 kPa)、中等(88 kPa)到硬基质(323 kPa)的不同硬度的胶原包被聚丙烯酰胺凝胶,发现硬基质上巨噬细胞是促炎表型,而软/中等刚度基质更易表达抗炎性、促修复的因子。 PREVITERA等[39]制备了0.3-230 kPa系列不同弹性模量的凝胶,考察了凝胶对巨噬细胞中Toll样受体4(Toll-like receptor4,TLR-4) 炎性信号中关键蛋白和转录因子表达量的影响,发现肿瘤坏死因子α、白细胞介素1β和一氧化氮等炎性因子浓度随着凝胶硬度的增加而增加,TLR4信号途径中TLR4、磷酸化核转录因子κB随着基材硬度(47-230 kPa)的增加而增加。这一现象在较低刚性(0.1,3.4,10.3 kPa)材料上的巨噬细胞行为中也得到验证[40]。此外,底物硬度对巨噬细胞的调节还涉及Yes相关蛋白/PDZ结合基序信号途径。刚性基底材料能够激活Yes 相关蛋白和具有PDZ结合基序的信号传导[41-43]。较硬的水凝胶促进了巨噬细胞的Yes相关蛋白高表达及核易位,增加巨噬细胞炎性反应[44]。 FENG等[45]发现促成骨的信号通路Wnt5a,通过激活Yes相关蛋白/ PDZ结合基序信号途径,促进巨噬细胞M2极化及转化生长因子β1的表达,从而诱导纤维化。制备较软的基底材料(1-5 kPa)是抗纤维化材料设计需考虑的一个因素[46]。由此可见,植入物生物材料的基材刚度越高,巨噬细胞引起的炎性反应越强,巨噬细胞更易转化为炎性表型,不利于组织修复。那么,在生物材料研究或者应用角度分析,研究者可以更倾向于设计并制备具有较小弹性模量的生物材料,如水凝胶等,以此类材料为基质材料或者实现植入物-组织接触面全覆盖,降低因植入物对损伤组织的应力传到,减少因巨噬细胞聚集后而引起的炎性因子表达,从而改善组织修复过程中的纤维化程度。 文章总结了生物材料物理学特性对其纤维化的作用相关研究进展,见表3。"
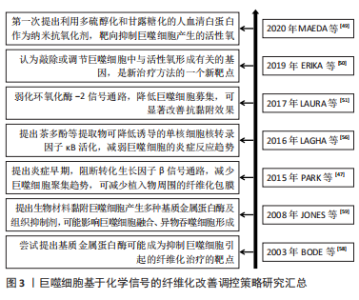
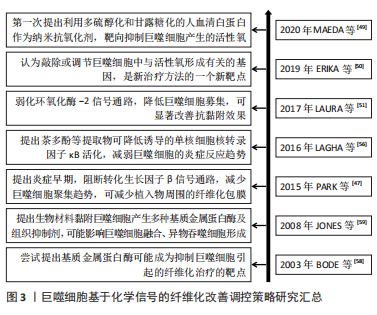
2.2.2 基于化学信号的调控策略 基于巨噬细胞招募的化学信号调控策略:转化生长因子β1等被认为是促纤维化的细胞因子,能募集宿主巨噬细胞。那么,转化生长因子β1等因子的抑制剂,可减少植入物的纤维化程度,从而实现更好的组织修复。PARK等[47]在硅胶植入物上喷涂了可以控制释放转化生长因子β1抑制剂曲尼司特的聚乳酸-乙醇酸涂层,显著减少了巨噬细胞数量并降低了纤维囊厚度。可见,药物对转化生长因子β1信号通路的早期抑制,可能减少了单核细胞募集,减少了成纤维细胞数量且纤维囊形成趋势减弱,由此导致愈合后期的纤维形成减弱。此外,LIU等[48]使用侧链上带有酯连接的布洛芬前药,与聚乳酸-乙醇酸结合制备静电纺丝生物膜,持续释放布洛芬,抑制大鼠椎板切除术后植入膜的巨噬细胞数量及炎症因子表达,起到抗纤维化作用,减少了相关的神经功能缺陷。可见,通过可控药物引起的持续化学作用,并结合载体材料的可控降解,对转化生长因子β1或者环氧化酶2信号通路的逐步抑制,可减缓或抑制巨噬细胞的促纤维化过程,甚至可能改善神经组织功能,帮助临床实现更优组织修复。 基于巨噬细胞来源炎症因子的化学信号调控策略:在刺激作用或者吞噬作用下,巨噬细胞可产生大量的活性氧,这也是活性氧的主要来源,能促进分泌基质金属蛋白酶、肿瘤坏死因子α、白细胞介素6和白细胞介素1β等炎性因子,启动炎性促纤维化途径。MAEDA等[49]以多硫醇化和甘露糖化的人血清白蛋白作为纳米抗氧化剂,可抑制氧化应激水平。该文献显示,可靶向抑制巨噬细胞产生的活性氧,从而缓解肝纤维化进程。有研究表明,巨噬细胞通过上调活性氧形成,进一步刺激和维持巨噬细胞中的促炎通路[50-51]。从文献分析可知,靶向抑制巨噬细胞产生的活性氧,可能有助于抑制炎症;同时,使用抗氧化剂(如维生素C)降低活性氧的作用,在体外研究显示出了令人满意的结果,但它们转化为临床实践的效果并不令人满意。这此过程中,抗氧化作用不理想,可能是由于器官摄取有限,或其他干扰,如药物、遗传和环境因素。那么,可能认为敲除或调节巨噬细胞中与活性氧形成有关的基因,被认为是新治疗方法的一个新靶点。同样,有研究认为氧化石墨烯也可作为抗氧化剂,并通过减少细胞内活性氧、下调M1表型和体外炎症细胞因子分泌,进而减轻巨噬细胞导致的炎症,减轻纤维化并改善心脏功能[52]。从该文献结果可知,以巨噬细胞的活性氧为靶点,利用抗氧化剂作为抑制活性氧的作用物质,同时还可实施分子功能化修饰,即可减缓组织纤维化程度,也可改善组织功能,这也是近期有关研究的方向。 基于巨噬细胞融合的化学信号调控策略:巨噬细胞融合成异物巨细胞,依赖于核转录因子κB信号途径[53]。MORRIS等[54]设计了靶向核转录因子κB和转化生长因子β1的多组分系统,以静电纺丝方式将核转录因子κB信号途径的抑制剂Bay 11-7082,以及转化生长因子β1抑制剂吡非尼酮,联合聚己内酯,制备成电纺薄膜。该文献体外释放实验表明,药物的缓释可在1周后抑制巨噬细胞融合成异物巨细胞,在4周内以剂量依赖性方式减少纤维化包裹,该文献作者考虑到异物巨细胞的形成和封装是使用生物材料实施组织修复的严重并发症。那么,探索基于3D打印技术的静电纺丝方式,可以尝试制备不同洗脱结构的基质材料,即可覆盖在植入物表面也可独立作为植入物,以满足不同时间尺度的有效化学信号传递需求,从而减少前维护趋势,这也是近期关于巨噬细胞融合信号调控的研究方向之一。 另外,ZHANG等[55]研究表明,以核转录因子κB诱导的分泌型胚胎碱性磷酸酶染色体整合的小鼠巨噬细胞RAW-Blue细胞,可检测到TLR4 mRNA和蛋白等炎性表达。分析该文献,外源性氧化剂可能在激活炎症表型中发挥作用,它增加了细胞内活性氧的产生和脂质过氧化,从而导致细胞内总抗氧化能力下降。LAGHA等[56]研究认为,茶多酚等提取物显示存在诱导降低单核细胞核转录因子κB活化趋势,由于核转录因子κB是炎症递质编码基因的关键调节因,利用茶多酚等提取物对巨噬细胞进行预处理后,可抑制炎性因子的表达。 此外,巨噬细胞融合成异物巨细胞涉及基质金属蛋白酶[57-58]。它可以降解细胞外基质内的结构成分,并在细胞表面产生细胞行为的改变,即促进黏附和迁移;或者改变随后的病理反应,即延缓异物反应和伤口愈合。同时,组织抑制剂可能存在与基质金属蛋白酶相反的作用。从JONES等[59]研究分析可知,根据材料表面化学,亲水/中性表面可能抑制巨噬细胞黏附,从而产生更多的基质金属蛋白酶,激活黏附的巨噬细胞或异物巨细胞。亲水/阴离子表面上常组织抑制剂的浓度更高,而基质金属蛋白酶保持不变。因此,药物抑制基质金属蛋白酶,可减少巨噬细胞融合,但不影响黏附,并以此作为抑制生物材料引起的纤维化的策略。 基于巨噬细胞分化的化学信号调控策略:SINHA等[20]研究发现miRNA-21是巨噬细胞向成纤维细胞的转变所必须的,它可抑制巨噬细胞可塑性,从而抑制向成纤维细胞样细胞的转变。由此可知,miRNA-21对于能够将髓系细胞转化为成纤维细胞样细胞,至关重要。同时,分析该文献,基于miRNA-21靶点,利用antago-miRNA-21局部递送,特异性敲除角质形成细胞中miRNA-21,可使得角质形成细胞分泌的miRNA-21减少趋势,从而抑制巨噬细胞转变为成纤维细胞样细胞。MENG等[60]研究认为,通过白喉毒素选择性清除髓系细胞,可以很大程度上消除阻塞肾脏中的巨噬细胞浸润和肌成纤维细胞,并减少肌成纤维细胞的积累和胶原沉积,揭示了炎症巨噬细胞在肌成纤维细胞和组织纤维化中的致病作用。通过文献分析,认为巨噬细胞可直接转分化为产生胶原的肌成纤维细胞的假设可能是成立的。而通过调控巨噬细胞向成纤维细胞的转变,有利于减缓炎症表现。 文章总结了巨噬细胞基于化学信号的纤维化改善调控策略事件的研究时间图,见图3。"
| [1] WITHEREL CE, ABEBAYEHU D, BARKER TH, et al. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv Healthc Mater. 2019;8(4):e1801451. [2] NOSKOVICOVA N, HINZ B, PAKSHIR P. Implant fibrosis and the underappreciated role of myofibroblasts in the foreign body reaction. Cells. 2021;10(7):1794. [3] VASSE GF, NIZAMOGLU M, HEIJINK IH, et al. Macrophage-stroma interactions in fibrosis: biochemical, biophysical, and cellular perspectives. J Pathol. 2021;254(4):344-357. [4] BORRIELLO F, LONGO M, SPINELLI R, et al. IL-3 synergises with basophil-derived IL-4 and IL-13 to promote the alternative activation of human monocytes. Eur J Immunol. 2015;45(7):2042-2051. [5] WHEELER KC, JENA MK, PRADHAN BS, et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. Plos One. 2018;13(1):e191040. [6] HAIDER N, BOSCA L, ZANDBERGEN H R, et al. Transition of macrophages to fibroblast-like cells in healing myocardial infarction. J Am Coll Cardiol. 2019;74(25):3124-3135. [7] HINZ B. The role of myofibroblasts in wound healing. Curr Res Transl Med. 2016;64(4):171-177. [8] NOSKOVICOVA N, SCHUSTER R, PUTTEN SV, et al. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-beta. Nat Biomed Eng. 2021;5(12):1437-1456. [9] SCHUSTER R, ROCKEL JS, KAPOOR M, et al. The inflammatory speech of fibroblasts. Immunol Rev. 2021;302(1):126-146. [10] DENG M, TAN J, HU C, et al. Modification of PLGA scaffold by MSC‐Derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF‐β‐induced protein. Advanced Healthcare Materials. 2020;13(9):2000353. [11] DONDOSSOLAE, HOLZAPFEL BM, ALEXANDER S, et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat Biomed Eng. 2016;1:7. [12] BANK RA, ZANDSTRA J, ROOM H, et al. Biomaterial encapsulation is enhanced in the early Stages of the foreign nody reaction during conditional macrophage depletion in transgenic macrophage fas-induced apoptosis mice. Tissue Eng Part A. 2017;23(19-20):1078-1087. [13] WITHEREL CE, SAO K, BRISSON BK, et al. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials. 2021;269:120667. [14] TAN RP, HALLAHAN N, KOSOBRODOVA E, et al. Bioactivation of encapsulation membranes reduces fibrosis and wnhances cell survival. ACS Appl Mater Interfaces. 2020;12(51):56908-56923. [15] KAPS L, LEBER N, KLEFENZ A, et al. In Vivo siRNA Delivery to immunosuppressive liver macrophages by alpha mannosyl functionalized cationic nanohydrogel particles. Cells. 2020;9(8):1905. [16] MCNALLY AK, ANDERSON JM. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: dependence on material surface properties. J Biomed Mater Res A. 2015;103(4):1380-1390. [17] MOONEY JE, ROLFE BE, OSBORNE GW, et al. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. Am J Pathol. 2010;176(1):369-380. [18] WANG S, MENG XM, NG YY, et al. TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget. 2016;7(8):8809-8822. [19] MENG XM, WANG S, HUANG XR, et al. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016;7(12):e2495. [20] SINHA M, SEN C K, SINGH K, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nature Communications. 2018;9(1):936. [21] LITTLE K, LLORIAN S M, TANG M, et al. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J Neuroinflammation. 2020;17(1):355. [22] TANG PCT, CHUNG JYF, XUE VWW, et al. Smad3 Promotes cancer associated fibroblasts generation via macrophage myofibroblast transition. Adv Sci. 2022;9(1):2101235. [23] BEZHAEVA T, GEELHOED WJ, WANG D, et al. Contribution of bone marrow derived cells to in situ engineered tissue capsules in a rat model of chronic kidney disease. Biomaterials. 2019;194:47-56. [24] CHAKRABORTY D, SUMOVA B, MALLANO T, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8(1):1130. [25] KNIPPER JA, WILLENBORG S, BRINCKMANN J, et al. Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity. 2015;43(4):803-816. [26] FICHTNER FS, STROBER W, KAWAKAMI K, et al. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99-106. [27] ZHANG B, ZHANG Z, XIA S, et al. KLF5 activates microRNA 200 transcription to maintain epithelial characteristics and prevent induced epithelial-mesenchymal transition in epithelial cells. Mol Cell Biol. 2013;33(24):4919-4935. [28] BOWEN KA, DOAN HQ, ZHOU BP, et al. PTEN loss induces epithelial--mesenchymal transition in human colon cancer cells. Anticancer Res. 2009;29(11):4439-4449. [29] LO CA, DELEVOYE C, GILLES MF, et al. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat Commun. 2015;6:7506. [30] KRICHEVSKY AM, GABRIELY G. MiR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39-53. [31] WANG YY, JIANG H, PAN J, et al. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol. 2017;28(7):2053-2067. [32] TORRES A, MUNOZ K, NAHUELPAN Y, et al. Intraglomerular Monocyte/Macrophage Infiltration and Macrophage-Myofibroblast Transition during Diabetic Nephropathy Is Regulated by the A2B Adenosine Receptor. Cells. 2020;9(4):1051. [33] WELLS RG. Tissue mechanics and fibrosis. Biochim Biophys Acta. 2013; 1832(7):884-890. [34] VASSE GF, NIZAMOGLU M, HEIJINK IH, et al. Macrophage-stroma interactions in fibrosis: biochemical, biophysical, and cellular perspectives. J Pathol. 2021;254(4):344-357. [35] SHAYAN M, PADMANABHAN J, MORRIS AH, et al. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization. Acta Biomater. 2018;75:427-438. [36] MOON H, CREMMEL CV, KULPA A, et al. Novel grooved substrata stimulate macrophage fusion, CCL2 and MMP-9 secretion. J Biomed Mater Res A. 2016;104(9):2243-2254. [37] MAJD H, SCHERER SS, BOO S, et al. Novel micropatterns mechanically control fibrotic reactions at the surface of silicone implants. Biomaterials. 2015;54:136-147. [38] SRIDHARAN R, CAVANAGH B, CAMERON AR, et al. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47-59. [39] PREVITERA ML, SENGUPTA A. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One. 2015;10(12):e145813. [40] SCOTT RA, KIICK KL, AKINS RE. Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages. Acta Biomater. 2021;122:220-235. [41] WEI Q, HOLLE A, LI J, et al. BMP-2 Signaling and Mechanotransduction Synergize to Drive Osteogenic Differentiation via YAP/TAZ. Adv Sci (Weinh). 2020;7(15):1902931. [42] KURODA M, UEDA K, KIOKA N. Vinexin family (SORBS) proteins regulate mechanotransduction in mesenchymal stem cells. Sci Rep. 2018;8(1):11581. [43] DUPONT S, MORSUT L, ARAGONA M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179-183. [44] MELI V S, ATCHA H, VEERASUBRAMANIAN PK, et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci Adv. 2020;6(49):eabb8471. [45] FENG Y, LIANG Y, ZHU X, et al. The signaling protein Wnt5a promotes TGFbeta1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J Biol Chem. 2018; 293(50):19290-19302. [46] BLAKNEY AK, SWARTZLANDER MD, BRYANT SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly (ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100(6):1375-1386. [47] PARK S, PARK M, KIM BH, et al. Acute suppression of TGF-ss with local, sustained release of tranilast against the formation of fibrous capsules around silicone implants. J Control Release. 2015;200:125-137. [48] LIU S, PAN G, LIU G, et al. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J Control Release. 2017;264:1-13. [49] MAEDA H, MINAYOSHI Y, ICHIMIZU S, et al. Repeated administration of Kupffer cells-targeting nanoantioxidant ameliorates liver fibrosis in an wxperimental mouse model. Biol Pharm Bull. 2020;43(1):93-101. [50] ERIKA R, VLADIMIR R, DIEUWERTJE M M, et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;2(224):242-253. [51] LAURA F, FULVIO S, CRISTINA NA, et al. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 2017;19:1202-1213. [52] HAN J, KIM YS, LIM MY, et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano. 2018;12(2):1959-1977. [53] KAJAL C, SOUMYA K, MINJU J. Polygalactan from bivalve Crassostrea madrasensis attenuates nuclear factor-κB activation and cytokine production in lipopolysaccharide-activated macrophage. Carbohyd Polym. 2020;249:116817. [54] MORRIS AH, MAHAL RS, UDELL J, et al. Multicompartment drug release system for dynamic modulation of tissue responses. Adv Healthc Mater. 2017. doi: 10.1002/adhm.201700370. [55] ZHANG Y, ORISA JI. Exogenous oxidants activate nuclear factor kappa B through Toll-like receptor 4 stimulation to maintain inflammatory phenotype in macrophage. Biochem Pharmacol. 2018;147:104-118. [56] LAGHA AB, GRENIER D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci Rep 2016;6:34520. [57] YUE L, SHI YJ, SU XP, et al. Matrix metalloproteinases inhibitors in idiopathic pulmonary fibrosis: medicinal chemistry perspectives. Eur J Med Chem. 2021;224:113714. [58] BODE W, MASKOS K. Structural basis of the matrix metalloproteinases and their physiological inhibitors, the tissue inhibitors of metalloproteinases. Biol Chem Hoppe Seyler. 2003;384(6):863-872. [59] JONES JA, MCNALLY AK, Chang DT, et al. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res A. 2008;84(1):158-166. [60] MENG XM , WANG S, HUANG XR, et al. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016;7(12):e2495. |
| [1] | Guo Shuhui, Yang Ye, Jiang Yangyang, Xu Jianwen. Screening and validation of neurogenic bladder miRNA-mRNA regulatory network [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-8. |
| [2] | Fang Xingyan, Tian Zhenli, Zhao Zheyi, Wen Ping, Xie Tingting. Effects of sodium arsenite on human umbilical vein endothelial cell injury and sphingosine kinases 1/sphingosine 1-phosphate signaling axis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-7. |
| [3] | He Yinhao, Li Xiaosheng, Chen Hongwen, Chen Tiezhu. 3D printed porous tantalum metal in the treatment of developmental dysplasia of the hip: current status and application prospect [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1455-1461. |
| [4] | Yang Zhishan, Tang Zhenglong. YAP/TAZ, a core factor of the Hippo signaling pathway, is involved in bone formation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1264-1271. |
| [5] | Liang Jiaqi, Liu Hengxu, Yang Jinxin, Yang Yi, Deng Xuhui, Tan Mingjian, Luo Jiong. Health benefit relationship between exercise and intestinal bacteria [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1292-1299. |
| [6] | Gao Yu, Han Jiahui, Ge Xin. Immunoinflammatory microenvironment after spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1300-1305. |
| [7] | Tang Liang, Li Xiheng, Niu Ruijuan, Li Xinyue, Zou Xinying, Mao Tianjiao, Li Jiang. Naringin regulates the function of RAW264.7 macrophages to affect the osteogenic differentiation of MC-3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1205-1210. |
| [8] | Yang Yitian, Wang Lu, Yao Wei, Zhao Bin. Application of the interaction between biological scaffolds and macrophages in bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1071-1079. |
| [9] | Xue Ting, Zhang Xinri, Kong Xiaomei. Mesenchymal stem cell therapy for pneumoconiosis using nanomaterials combined with multi-modal molecular imaging [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1133-1140. |
| [10] | Li Wenjie, You Aijia, Zhou Junli, Fang Sujuan, Li Chun. Effects of different dressings in the treatment of burn wounds: a network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1141-1148. |
| [11] | Liu Wentao, Feng Xingchao, Yang Yi, Bai Shengbin. Effect of M2 macrophage-derived exosomes on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 840-845. |
| [12] | Wang Min, Yin Xiushan, Wang Yingxi, Zhang Yan, Zhao Long, Xia Shuyue. Inhalation of bone marrow mesenchymal stem cells-derived exosomes alleviates inflammatory injury in chronic obstructive pulmonary disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 827-834. |
| [13] | Zhang Qijian, Xu Ximing. Acquisition and application of ectodermal mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 928-934. |
| [14] | Xu Qijing, Yang Yichun, Lei Wei, Yang Ying, Yu Jiang, Xia Tingting, Zhang Meng, Zhang Tao, Zhang Qian. Advances and problems in cell-free treatment of diabetic skin chronic wounds [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 962-969. |
| [15] | Li Zhichao, Tan Guoqing, Su Hui, Xu Zhanwang, Xue Haipeng. Regulatory role of non-coding RNAs as potential therapeutic targets in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 758-764. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||