Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (8): 1292-1299.doi: 10.12307/2023.087
Previous Articles Next Articles
Health benefit relationship between exercise and intestinal bacteria
Liang Jiaqi, Liu Hengxu, Yang Jinxin, Yang Yi, Deng Xuhui, Tan Mingjian, Luo Jiong
- Sports Addiction Research Center, School of Physical Education, Southwest University, Chongqing 400715, China
-
Received:2022-02-22Accepted:2022-04-28Online:2023-03-18Published:2022-07-29 -
Contact:Luo Jiong, MD, Professor, Doctoral supervisor, Sports Addiction Research Center, School of Physical Education, Southwest University, Chongqing 400715, China -
About author:Liang Jiaqi, Master, Sports Addiction Research Center, School of Physical Education, Southwest University, Chongqing 400715, China -
Supported by:the Fundamental Research Funds for the Central Universities, No. SWU2009103 (to LJ)
CLC Number:
Cite this article
Liang Jiaqi, Liu Hengxu, Yang Jinxin, Yang Yi, Deng Xuhui, Tan Mingjian, Luo Jiong. Health benefit relationship between exercise and intestinal bacteria[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1292-1299.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

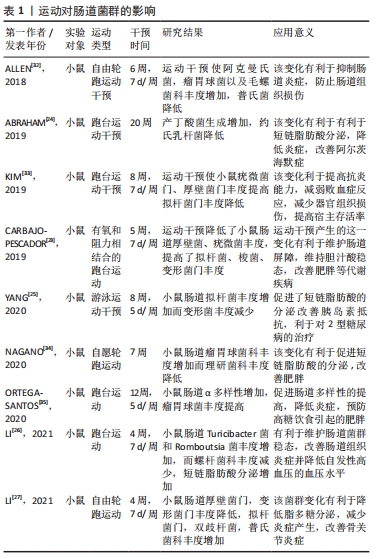
2.1 运动与肠道菌群的相关性 相关研究表明,肠道组织菌群与健康的联系越来越紧密,这些肠道菌通过参与机体组织的代谢、免疫、炎症反应以及氧化应激等机制,可以影响机体肠道屏障功能,防止病原体入侵,维护机体健康[15],此结论与以往研究观点一致[16-17],肠道菌群的组成、结构、功能及多样性的扰动将对人体的能量代谢、免疫、炎症以及氧化应激等产生重要影响。当人体在长时间的大负荷量运动中,将使人体胃肠道血流量降低,从而引起免疫系统发生反应,肠道菌比例失调,有害菌增多,使脂多糖生成增加,这导致肠道上皮组织黏膜细胞遭到破坏,引起胃肠道疾病和机体组织低度炎症的发生,从而对机体各组织健康以及运动表现产生不良影响[18-19]。然而有研究报道肠道菌可以影响机体健康,运动者可通过益生菌的补充来维持肠道菌群稳定,从而提高肠道免疫力,防止肠道上皮黏膜组织细胞损伤,改善运动过程中运动者出现的机体组织炎症及上呼吸道感染等疾病[20-21]。 运动对肠道菌群的影响在以下研究中得以证实。在JANABI等[22]研究中发现,运动对马肠道菌群有显著调节作用,该研究将马分为运动组和季节对照组,在3个月运动干预后运动组马体内肠道菌群丰度发生明显变化,其中拟杆菌门和变形菌门丰度增加,而对照组肠道菌未发生明显改变。而在CARBAJO-PESCADOR等[23]学者的动物研究中,对高脂饮食小鼠进行有氧和阻力运动干预,发现运动使得小鼠肠道厚壁菌及疣微菌等丰度降低,拟杆菌、梭菌和变形菌等丰度增加,该研究提示,通过对肠道组成和功能的调节可在一定程度上改善代谢性综合征等。同样,在Abraham团队的动物实验中,对雄性APP/PS1TG小鼠进行跑台运动干预,发现运动干预改善了肠道微生物群,使丁酸产生菌增加,并减少了约氏乳杆菌等,该变化有利于改善机体组织炎症,降低结肠癌发病率等[24]。YANG等[25]在动物实验中对2型糖尿病小鼠进行为期8周每周5 d的游泳运动干预,运动干预使小鼠菌群得到重塑,相关短链脂肪酸生成菌丰度增加,胰岛素敏感性得到改善,有利于2型糖尿病的治疗。与YANG等[25]的实验相比,LI等[26]实验中对小鼠进行4周每周7 d的跑台运动干预,干预使得肠道中Turicibacter菌和Romboutsia菌的丰度增加,而螺杆菌科丰度减少,该变化改善了大鼠肠道组织炎症状况,并提高了其机体组织免疫力和抗高血压能力。LI等[27]对小鼠进行4周自由轮转运动干预,这使小鼠肠道中厚壁菌门和变形菌门丰度降低而拟杆菌,双歧杆菌及普氏菌丰度上升,该变化有利于维持肠道菌群稳态,减少脂多糖的分泌,维护肠道屏障功能,进而降低小鼠机体组织炎症促进骨关节炎的改善。 运动改善肠道菌群对机体健康的影响不仅在动物实验中得以体现,在相关人体运动干预研究中也有所展现。据CODELLA等[28]的研究得知,运动可以改善多种慢性疾病,例如糖尿病及胃肠道疾病等,但运动改善疾病主要是通过肠道菌间接实现的,通过规律性的运动可以增加肠道菌群多样性,促进机体炎症因子的减少,提高机体各组织抗炎能力。如在ROGERO等[29]在研究中表明,肠道菌群对肥胖的产生有着极为重要的影响,肥胖导致的炎症等反应会引起糖尿病及心血管疾病等,它是多种疾病的诱因。而在QUIROGA等[30]的研究中,通过对7-12岁肥胖儿童进行为期12周的力量和耐力联合运动干预,显著改变了肠道菌群的组成和功能,使肠道中梭菌、变形菌及拟杆菌门的相对丰度发生改变,促进了肠道稳态,并使得脂多糖分泌水平下降,从而降低肠道通透性,减少相关组织炎症,改善肥胖综合征,有利于促进人体健康。同样,运动干预效果在老年女性志愿者中也得以展现,MORITA等[31]对老年女性志愿者进行12周有氧运动干预使得肠道拟杆菌丰度也显著提高。 上述研究结果提示,运动与肠道菌群具有有明显的相关性,这在人体和动物实验得以证实。运动与肠道菌群相互影响,通过益生菌的补充可以促进肠道菌群稳态,有利于缓解机体在运动过程中及运动结束后产生的一系列健康问题。相反,通过规律性运动可介入肠道菌群,改变其组成、功能、结构、丰度及多样性等,这会对机体组织炎症、免疫、能量代谢以及氧化应激等产生积极重要影响,进而可以达到预防或改善相关疾病、促进机体各组织健康的目的,具体研究成果见表1。 "
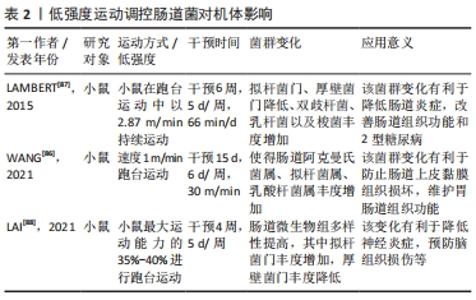
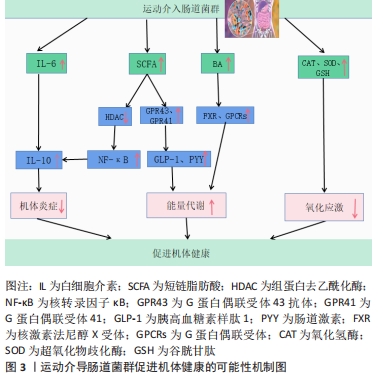
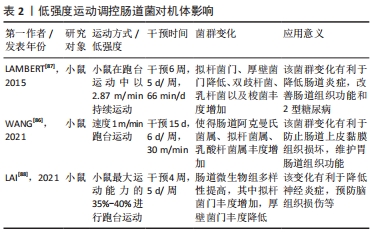
2.2.1 运动介入肠道菌对宿主炎症反应的影响 运动具有重要的抗炎作用,然而运动的抗炎作用主要通过干预肠道菌群得以实现[36]。根据相关研究发现,当肠道菌群紊乱时,将使肠道菌多样性和稳定性遭到破坏,导致革兰阴性菌生成的增加,这使脂多糖大量分泌,当脂多糖透过肠道组织屏障进入血液与内毒素结合蛋白相结合,将造成肠道上皮组织黏膜细胞损害,使肠道通透性增加,引起宿主机体组织产生炎症[37-38]。然而当机体全身产生低度炎症时,体内巨噬细胞中的Toll样受体及核转录因子κB将被激活[39],Toll样受体能够识别细胞细菌表达基元,Toll样受体的激活启动信号级联,涉及蛋白质和转录因子的激活,通过一系列转导过程使胃肠道中促炎因子白细胞介素6、肿瘤坏死因子、转化生长因子β以及相关细胞因子大量分泌,导致机体组织炎症,进而使胰岛素敏感性降低导致糖尿病等[40-42]。 据上文研究可知运动对肠道菌群有着极强的重塑作用。然而肠道菌群变化将显著影响炎症因子白细胞介素6和其自身代谢产物短链脂肪酸的分泌,从而影响机体抗炎能力[43-44]。在MAHDIEH等[45]的实验中,对肥胖女性志愿者进行10周中到高强度有氧运动训练,发现运动干预能改变肥胖女性肠道中双歧杆菌分泌,从而可以对炎症因子白细胞介素6产生影响。据相关研究显示,肠道菌群中双歧杆菌与乳杆菌与白细胞介素6呈负相关,如这2个菌株在肠道中大量减少将导致血液中的白细胞介素6含量剧增[46],而血液中白细胞介素6的大量发现,将抑制肿瘤坏死因子α并激活抗炎反应的细胞激素如白细胞介素1ra和白细胞介素10的释放,从而达到降低宿主全身炎症的目的[47]。另外,运动可以介入肠道菌群促使短链脂肪酸生成增加,这将会加强对巨噬细胞和树突细胞中的组蛋白去乙酰化酶的抑制,使核转录因子κB的活性降低,以及中性粒细胞和巨噬细胞分泌的促炎因子如白细胞介素6、白细胞介素8和肿瘤坏死因子α减少,进而促进抗炎因子白细胞介素10等增加,改善机体健康[25,48-50]。 上述研究证实,运动介入肠道菌群可以促进相关抗炎因子白细胞介素10增加,并使短链脂肪酸分泌增多,这有利于白细胞介素6和白细胞介素8等相关促炎因子的降低,从而改善宿主机体组织炎症,促进宿主健康。由此可知,肠道菌群可以成为运动改善机体组织炎症的重要靶点,改善相关疾病的发生与发展。 2.2.2 运动通过肠道菌群对宿主能量代谢的影响 能量代谢在运动中及运动外对机体健康均有着重要的影响。能量代谢是影响机体健康的重要因素之一,一旦代谢平衡遭到破坏,将会导致相应疾病产生,如代谢性疾病、神经性疾病、心血管疾病以及癌症等[51-52]。其次,根据VILLA等[53]的研究可知,在运动中能量代谢也是影响运动表现的重要因素。由于机体在运动中为了向肌肉组织持续收缩时的基本细胞提供ATP,机体会根据运动强度和运动持续时间,通过不同能量代谢机制来确保ATP再合成,而这一感知反应将快速通过相应的信号传导途径使能量代谢机制间相互协调,从而促进细胞能量代谢平衡的特定分子的合成,以满足肌肉组织在运动时对ATP的需求[54]。然而当人体在高强度剧烈运动状态下,如果由于各种原因导致长时间能量供应不足,这必将会损害人体免疫功能,增加人体呼吸道等疾病的易感性,影响人体生理和心理健康[55]。运动中如果通过糖酵解供能系统为机体供能,此过程将会产生丙酮酸,丙酮酸无法通过氧化磷酸化过程在线粒体内被完全氧化,将被乳酸脱氢酶转化为乳酸,然而肌内酸中毒可能引起机体疲劳,导致肌肉能量代谢紊乱[54,56]。 运动对肠道菌群具有显著的调控作用,而肠道微生物群在宿主机体组织代谢中起着基础性作用[57]。肠道微生物群可以通过调节其代谢产物影响宿主机体组织能量代谢。有研究表明,规律性运动可以调节肠道微生物群的组成,改善肠道黏膜屏障组织功能,提高拟杆菌与厚壁菌门的比例,改变胆汁酸谱并提高短链脂肪酸的生成量[58]。在FUNABASHI等[59]的研究中显示,胆汁酸是肠道微生物群的代谢产物之一。有研究在实验中对约4周龄的C57BL/6高脂饮食小鼠进行12周中等强度跑台运动干预,发现运动干预会引起胆汁酸相关产菌(厚壁菌)丰度显著增加,这可使胆汁酸分泌增多[35]。肠道微生物群通过和初级胆汁酸(胆酸和鹅去氧胆酸)之间的相互作用可以生成次级胆汁酸(脱氧胆酸和石)[60]。这表明肠道菌群可以直接影响次级胆汁酸的分泌。在LI等[61]学者研究中表明,胆汁酸可以促进膳食脂质和脂溶性维生素的消化和吸收,从而影响人体能量的摄取。其次有相同研究显示,胆汁酸是重要的信号分子,它可以激活人体器官中的核激素法尼醇X受体(Farnesoid X Receptor,FXR)和G蛋白偶联受体(G protein-coupled receptor,GPCRs)从而调节宿主机体脂质,葡萄糖和能量代谢的稳态[62]。MA等[40]研究得出,如果肠道菌群紊乱将导致次级胆汁酸生成减少,使FXR和G蛋白偶联受体1(G protein-coupled receptor 1,TGR5)受体激活减少进而导致机体组织能量代谢的紊乱。 然而对能量代谢有显著影响的还有短链脂肪酸,短链脂肪酸是碳水化合物通过肠道菌群发酵产生的重要代谢产物之一,它主要分为丁酸盐、醋酸盐及丙酸盐等[63]。有研究发现,短链脂肪酸的产生菌有双歧杆菌、乳杆菌、真杆菌及瘤胃球菌等,其主要是由肠道拟杆菌发酵产生[64-65]。在YANG等[25]的实验中,用高脂饮食小鼠建立2型糖尿病小鼠模型,并对小鼠进行的游泳运动干预,发现运动干预使肠道拟杆菌丰度显著增加,从而使得2型糖尿病小鼠体内短链脂肪酸含量增加,而短链脂肪酸可以通过激活游离脂肪酸受体3来刺激内分泌细胞分泌肠道多肽YY激素(Peptide YY hormone,PYY)[66],还可通过激活游离脂肪酸受体2来刺激胰高血糖素样肽1(Glucagon-like peptide-1,GLP-1)以及瘦素的分泌[67],PYY和GLP-1可以改善肠道蠕动功能,提高机体组织对营养吸收和能量摄取的水平,而瘦素可以直接促进肝糖原的合成和肌肉血糖的摄取[68-69]。 上述研究提示,运动可以影响机体组织能量代谢水平,这主要通过运动介导肠道菌群调节其代谢产物而产生的影响。在科学的运动干预下可使胆汁酸和短链脂肪酸分泌水平呈上升趋势,这一变化利于提高机体能量代谢水平,维持能量代谢平衡等。 2.2.3 运动调节肠道菌对宿主氧化应激的影响 运动的主要生理作用之一是调节氧化应激和亚硝化应激,从而预防病原体的感染和组织损伤,并缓解机体组织炎症[70-71]。据WEISS等[72]的研究报道,氧化应激是由过度积累的活性氧和细胞抗氧化能力不平衡而导致的,而HU等[73]学者认为氧化应激是由于肠道微生物失调以及相关特定细菌的滋生增多而导致生态失衡的一个因素。活性氧物质(reactive oxygen species,ROS)和活性氮物质(reactive nitrogen species,RNS)的核心来源是肠道,它们是由肠道细胞和微生物群代谢的正常产物[74]。LAURIDSEN等[75]报道了ROS对人体健康有重要影响,ROS在细胞信号通路传导中有重要的调节作用,它不仅可以诱导有丝分裂反应,还可以防止病原体入侵。然而当ROS浓度超过体内正常水平将会导致肌肉细胞膜,脂质和蛋白质过氧化从而产生氧化应激[76]。据新近研究报道,抗氧化酶系统[超氧化物歧化酶(SOD),谷胱甘肽过氧化物酶(GPX),氧化氢酶(CAT)]和非酶系统[谷胱甘肽(GSH),硫氧还蛋白]对ROS和RON具有调控和消除作用[77-78]。 肠道菌群对氧化还原及平衡有着重要的影响,当肠道菌群紊乱,致病菌会过度增长,使肠道上皮组织细胞紧密性遭到破坏,导致脂多糖进入血液导致氧化应激[77],而调节肠道菌群组成、结构会改善氧化应激[79]。在胃肠道中乳杆菌和双歧杆菌与抗氧化能力呈正相关[80],因此通过增加乳杆菌和双歧杆菌,可以维持肠道酸碱平衡,促进肠道微生物保持稳态,减少致病菌的产生,保护肠道上皮组织黏膜屏障,从而减少氧化应激发生。有学者在小鼠实验研究中,对雄性小鼠进行自由轮转运动干预,发现运动干预使得血清瘦素浓度增加,肠道中乳杆菌、双歧杆菌以及普氏菌等数量显著增加[81]。然而,ZHAO等[82]使用D-半乳糖对小鼠氧化衰老进行诱导,通过检测发现乳杆菌可以增加超氧化物歧化酶、谷胱甘肽、氧化氢酶等抗氧化酶和非酶抗氧化系统活性。其次,SANCHEZ MACARRO等[83]的研究对健康男性志愿者进行高强度自行车滚轮运动干预,诱导氧化应激的产生,并通过双歧杆菌和乳杆菌进行改善,发现通过这两菌株可以使氧化应激生物标志物降低。同样,WANG等[84]实验结果表明双歧杆菌和乳杆菌可显著抑制血管紧张素Ⅱ,并保持细胞活力不变,还可通过提高细胞内氧化氢酶和超氧化物歧化酶活力防止ROS的增加。这些研究提示,肠道菌群对氧化应激有着重要的影响,运动可调节肠道菌群中的双歧杆菌和乳杆菌分泌量,间接地参与到改善氧化应激的过程当中。乳杆菌和双歧杆菌可以显著调节谷胱甘肽、超氧化物歧化酶、氧化氢酶等抗氧化酶和非酶抗氧化系统能力,来调控或消除氧化应激和亚硝化应激。由此,制定科学的运动方案可以作为一种非药物治疗方式,参与到肠道菌群改善氧化应激过程中,从而减少宿主病菌的感染以及机体组织的损伤。 2.3 不同运动强度通过肠道菌对宿主健康的影响 运动对肠道菌群有重要的调控作用[85],然而不同运动强度干预肠道菌对机体健康的影响还值得研究分析。以下,将根据目前有限文献对低-中-高3种运动强度干预肠道菌影响健康的状况进行总结。 WANG等[86]通过低强度跑步机运动对肠道辐射损伤小鼠进行运动干预,发现运动可以使小鼠肠道中拟杆菌、阿克曼氏菌、乳杆菌丰度增加,阿克曼氏菌和乳酸杆菌在预防及治疗肠道组织炎症和损伤中发挥着重要的作用,这一变化有助于保护肠道上皮黏膜组织,使肠道组织结构和功能得到重塑,维护肠道组织健康。LAMBERT等[87]学者对2型糖尿病小鼠进行低强度跑台运动干预,通过对干预前后粪便菌群分布发现,跑步机运动使得2型糖尿病小鼠肠道拟杆菌门及厚壁菌门丰度降低,双歧杆菌增加。双歧杆菌与乳杆菌相似,都是人体质量要的有益菌,它可以抑制致病菌的产生和机体组织炎症,维护肠道黏膜物理屏障功能,保护胃肠道组织,防止内毒素血症等,详见表2。 "
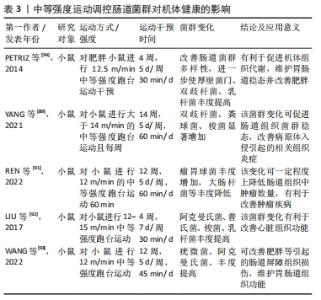
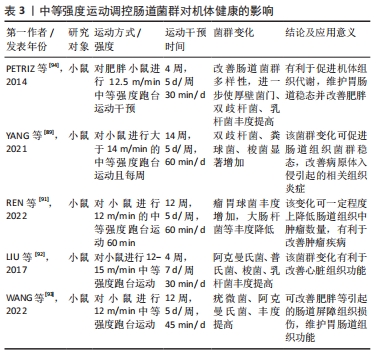
在中等强度运动状态下,肠道菌群变化对机体健康的影响与低强度运动干预结果较为一致。中等强度运动干预通过肠道菌群对机体健康产生有益影响。在相关研究中,将小鼠分为运动和非运动组,运动组小鼠进行14周中等强度跑台运动,干预后将非运动组和运动组小鼠肠道菌群进行比较,发现运动组双歧杆菌、粪球菌、乳杆菌、梭菌等显著高于非运动组小鼠,而双歧杆菌、梭菌和乳杆菌等可以防止病原体入侵,促进机体免疫力的提高,抑制有害菌产生,从而促进肠道组织稳态,调节机体组织炎症反应,预防疾病产生[89-90]。同时,REN等[91]用4周龄C57BL/6J小鼠建立ApcMin/+小鼠模型,并对肿瘤患病小鼠进行12周中等强度的有氧运动干预,发现运动干预后使肿瘤患病小鼠肠道菌群组成和丰度发生变化,其中瘤胃球菌科等益生菌丰度增加而埃希氏菌属等致病菌丰度减少,这表明该变化对肿瘤疾病有一定改善作用。LIU等[92]在实验中对C57BL/6J小鼠进行4周中等强度运动干预,发现运动后小鼠和对照组小鼠肠道菌群存在明显差异,其中运动组阿克曼氏菌、普氏菌属、梭菌及乳杆菌丰度得到明显增加,该变化有利于机体心脏组织功能的改善,促进机体健康,详见表3。 "

然而,当前高强度运动干预肠道菌群对健康产生影响的相关报道较少,但也有一些不同研究观点。传统观点认为高强度运动对机体健康有害,而YUAN等[95]将6周龄雄性小鼠进行4周高强度游泳训练,该运动训练使得小鼠肠道菌群多样性整体降低,其中有益菌丰度降低而致病菌生成增加,对机体组织免疫及代谢功能产生负面影响,使组织遭到损害,并导致内脏损伤。但DENOU等[96]研究结论与YUAN等[95]观点不同,该研究通过对雄性C57BL/6小鼠进行高质饮食诱导,对诱导的高脂饮食小鼠进行高强度间歇跑台运动,发现高强度间歇跑台运动使小鼠肠道拟杆菌分泌增加,拟杆菌与厚壁菌比率上升,且肠道α多样性也相应增加,该研究表明高强度间歇跑台运动在一定程度上可以克服由饮食引起的肥胖等一系列代谢疾病导致的肠道菌群紊乱,并对相关疾病有一定改善作用[96]。此外,BARTONW等[97]将职业橄榄球运动员与久坐不动者肠道菌群相比较,发现职业运动员肠道菌多样性明显高于久坐不动者,而运动员代谢相关的微生物基因的丰度也相对更高,其次阿克曼氏菌(Akkermansia)等有益菌的比例也明显高于低体质量指数对照组,职业运动员肠道菌群代谢产物短链脂肪酸短链脂肪酸的产量比久坐不动组的更为丰富,运动员主要以高强度剧烈运动为主,因此可知长期高强度运动可能使有益肠道菌丰度的增加,以降低各组织炎症促进肌肉组织损伤的恢复。 因此,低-中强度运动可明显促进阿克曼氏菌、乳杆菌、双歧杆菌等益生菌丰度增加,从而促进肠道组织稳态,预防或改善相关疾病的发生与发展。然而当下对高强度运动介入肠道菌群是否能够促进机体组织健康还存明显争议,这还可能受运动模式、运动方式、运动频率、饮食摄入、休息时间、性别和年龄等相关因素影响。目前鲜有文献关于不同运动强度下肠道菌对机体组织健康的相关机制报道,这值得学者进一步关注与探讨。 "

| [1] ÁLVAREZ-MERCADO AI, NAVARRO-OLIVEROS M, ROBLES-SÁNCHEZ C, et al. Microbial population changes and their relationship with human health and disease. Microorganisms. 2019;7(3):68. [2] WU GD, LEWIS JD. Analysis of the human gut microbiome and association with disease. Clin Gastroenterol Hepatol. 2013;11(7):774-777. [3] QIN J, LI R, RAES J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59-65. [4] KIM S, COVINGTON A, PAMER EG. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017; 279(1):90-105. [5] REN Z, FAN Y, LI A, et al. Alterations of the human gut microbiome in chronic kidney disease. Adv Sci (Weinh). 2020;7(20):2001936. [6] LI C, PI G, LI F. The role of intestinal flora in the regulation of bone homeostasis. Front Cell Infect Microbiol. 2021;11:579323. [7] 谢国群,张院辉,郭晓冬,等.基于高通量测序技术的胰腺癌湿热证患者肠道菌群结构差异与功能变异研究[J].上海中医药杂志, 2019,53(12):9-16. [8] ZHU Q, JIANG S, DU G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiologyopen. 2020;9(8):e1053. [9] PASCALE A, MARCHESI N, MARELLI C, et al. Microbiota and metabolic diseases. Endocrine. 2018;61(3):357-371. [10] GUI HX, HU YN, LI JW, et al. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid Med Cell Longev. 2018;2018:8930374. [11] NIE Y. Dietary nutrition and intestinal flora: promising target for the treatment of disease. Food Sci Tech Trends. 2018;75:72-80. [12] SINGH RK, CHANG HW, YAN D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017; 15(1):73. [13] PEDERSEN BK, SALTIN B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25 Suppl 3:1-72. [14] MAILING LJ, ALLEN JM, BUFORD TW, et al. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. 2019;47(2):75-85. [15] MOHR AE, JAGER R, CARPENTER KC, et al. The athletic gut microbiota. J Int Soc Sports Nutr. 2020;17(1):24. [16] GOMES AC, HOFFMANN C, MOTA JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes. 2018;9(4):308-325. [17] TICINESI A, NOUVENNE A, CERUNDOLO N, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. 2019;11(7):1633. [18] DE OLIVEIRA EP, BURINI RC, JEUKENDRUP A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med. 2014;44 Suppl 1(Suppl 1):S79-S85. [19] JAGER R, MOHR AE, GARPENTER KC, et al. International society of sports nutrition position stand: probiotics. J Int Soc Sports Nutr. 2019;16(1):62. [20] SALARKIA N, GHADAMLI L, ZAERI F, et al. Effects of probiotic yogurt on performance, respiratory and digestive systems of young adult female endurance swimmers: a randomized controlled trial. Med J Islam Repub Iran. 2013;27(3):141-146. [21] WEST NP, PYNE DB, PEAKE JM, et al. Probiotics, immunity and exercise: a review. Exerc Immunol Rev. 2009;15:107-126. [22] JANABI AHD , BIDDLE AS, KLEIN D, et al. Exercise training-induced changes in the gut microbiota of Standardbred racehorses. Comp Exerc Physiol. 2016;12(3):119-130. [23] CARBAJO-PESCADOR S, PORRAS D, GARCIA-MEDIAVILLA MV, et al. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis Model Mech. 2019; 12(5):dmm039206. [24] ABRAHAM D, FEHER J, SCUDERI GL, et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: role of microbiome. Exp Gerontol. 2019;115:122-131. [25] YANG L, LIN H, LIN W, et al. Exercise ameliorates insulin resistance of type 2 diabetes through motivating short-chain fatty acid-mediated skeletal muscle cell autophagy. Biology. 2020;9(8):203. [26] LI Y, ZAFAR S, SALIH LBRAHIM RM, et al. Exercise and food supplement of vitamin C ameliorate hypertension through improvement of gut microflora in the spontaneously hypertensive rats. Life Sci. 2021;269:119097. [27] LI K, LIU A, ZONG W, et al. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J Biol Sci. 2021;28(1):40-49. [28] CODELLA R, LUZI L, TERRUZZI L. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis. 2018;50(4):331-341. [29] ROGERO MM, GALDER PC. Obesity, inflammation, Toll-like receptor 4 and fatty acids. Nutrients. 2018;10(4):432. [30] QUIROGA R, NISTAL E, ESTÉBANEZ B, et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med. 2020;52(7):1048-1061. [31] MORITA E, YOKOYAMA H, LMAI D, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients. 2019;11(4):868. [32] ALLEN JM, MAILING LJ, COHRS J, et al. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes. 2018;9(2):115-130. [33] KIM D, KANG H. Exercise training modifies gut microbiota with attenuated host responses to sepsis in wild-type mice. FASEB J. 2019; 33(4):5772-5781. [34] NAGANO T, YANO H. Effect of dietary cellulose nanofiber and exercise on obesity and gut microbiota in mice fed a high-fat-diet. Biosci Biotechnol Biochem. 2020;84(3):613-620. [35] ORTEGA-SANTOS CP, AL-NAKKASH L, WHISNER CM. Exercise and/or genistein treatment impact gut microbiota and inflammation after 12 weeks on a high-fat, high-sugar diet in C57BL/6 mice. Nutrients. 2020;12(11):3410. [36] WOSINSKA L, COTTER PD, O’SULLIVAN O, et al. The potential impact of probiotics on the gut microbiome of athletes. Nutrients. 2019;11(10):2270. [37] WANG W, LI Q, CHAI W, et al. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Sci Nutr. 2019;7(8):2636-2646. [38] FERRO D, BARATTA F, PASTORI D, et al. New insights into the pathogenesis of non-alcoholic fatty liver disease: gut-derived lipopolysaccharides and oxidative stress. Nutrients. 2020;12(9):2762. [39] ITO N, TSUJIMOTO H, UENO H, et al. Helicobacter pylori-Mediated Immunity and Signaling Transduction in Gastric Cancer. J Clin Med. 2020;9(11):3699. [40] MA Q, LI Y, LI P, et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharmacother. 2019;117:109138. [41] YEO JC, WALL AA, LUO L, et al. Distinct roles for APPL1 and APPL2 in regulating toll-like receptor 4 signaling in macrophages. Traffic. 2016; 17(9):1014-1026. [42] 袁和秀.肠道菌群失调对代谢综合征儿童免疫功能及血清炎症因子的影响[J].中国医学创新,2020,17(24):116-119. [43] SOWAH SA, RIEDI L, DAMMS-MACHADO A, et al. Effects of weight-loss interventions on short-chain fatty acid concentrations in blood and feces of adults: a systematic review. Adv Nutr. 2019;10(4):673-684. [44] SMITH RP, EASSON C, LYIE SM, et al. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One. 2019;14(10): e0222394. [45] MAHDIEH MS, MARYAM J, BITA B, et al. A pilot study on the relationship between Lactobacillus, Bifidibactrium counts and inflammatory factors following exercise training. Arch Physiol Biochem. 2021. doi: 10.1080/13813455.2021.1871763. [46] 裴光德,熊曼,杨小丽.乙型肝炎肝硬化患者肠道菌群变化与血清细胞因子水平的相关性[J].中国微生态学杂志,2021,33(9):1078-1081. [47] PETERSEN AM, PEDERSEN BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154-1162. [48] Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332-1345. [49] TAN J, MCKENZIE C, POTAMITIS M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [50] LIU L, LI Q, YANG Y, et al. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front Vet Sci. 2021;8:736739. [51] ZHOU R, GOU Q, XIAO Y, et al. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021;9(1):25. [52] ZHANG BY, LIU AL, GUAN-HUA DU. Energy metabolism disorder and diseases: from effects to potential targets. Acta Pharmaceutica Sinica. 2019. doi:10.16438/J.0513-4870.2019-0161. [53] VILLA M, VILLA-VICENTE JG, SECO-CALVO J, et al. Body composition, dietary intake and the risk of low energy availability in elite-level competitive rhythmic gymnasts. Nutrients. 2021;13(6):2083. [54] MOGHETTI P, BACCHI E, BRANGANI C, et al. Metabolic effects of exercise. Front Horm Res. 2016;47:44-57. [55] LOGUE D, MADIGAN SM, DELAHUNT E, et al. Low energy availability in athletes: a review of prevalence, dietary patterns, physiological health, and sports performance. Sports Med. 2018;48(1):73-96. [56] LANCHA JUNIOR AH, PAINELLU VDE S, SAUNDERS B, et al. Nutritional strategies to modulate intracellular and extracellular buffering capacity during high-intensity exercise. Sports Med. 2015;45 Suppl 1:S71-S81. [57] BI, RUOHONG, JIE GAO, et al. Progress in the treatment of diabetes mellitus based on intestinal flora homeostasis and the advancement of holistic analysis methods. Nat Product Commun. 2020. doi.org/10.1177/1934578X20918418. [58] DE SIRE A, DE SIRE R, PETITO V, et al. Gut-joint axis: the role of physical exercise on gut microbiota modulation in older people with osteoarthritis. Nutrients. 2020;12(2):574. [59] FUNABASHI M, GROVE TL, WANG M, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020; 582(7813):566-570. [60] JIAO N, BAKER SS, CHAPA-RODRIGUEZ A, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67(10):1881-1891. [61] LI T, APTE U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol. 2015;74:263-302. [62] SHAPIRO H, KOLODZIEJCZYK AA, HALSTUCH D, et al. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215(2): 383-396. [63] HU J, LIN S, ZHENG B, et al. Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr. 2018;58(8):1243-1249. [64] LEBLANC JG, CHAIN F, MARTÍ N R, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16(1):79. [65] LOUIS P, FLINT HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29-41. [66] LARRAUFIE P, MARTIN-GALLAUSIAUX C, LAPAQUE N, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8(1):74. [67] HE J, ZHANG P, SHEN L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356. [68] STEINERT RE, FEINLE-BISSET C, ASARIAN L, et al. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol Rev. 2017; 97(1):411-463. [69] D’SOUZA AM, NEUMANN UH, GLAVAS MM, et al. The glucoregulatory actions of leptin. Mol Metab. 2017;6(9):1052-1065. [70] LAURIDSEN C. From oxidative stress to inflammation: redox balance and immune system. Poult Sci. 2019;98(10):4240-4246. [71] EVANS LW, OMAYE ST. Use of saliva biomarkers to monitor efficacy of vitamin C in exercise-induced oxidative stress. Antioxidants. 2017; 6(1):5. [72] WEISS GA, HENNET T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74(16):2959-2977. [73] HU Y, CHEN D, ZHENG P, et al. The bidirectional interactions between resveratrol and gut microbiota: an insight into oxidative stress and inflammatory bowel disease therapy. Biomed Res Int. 2019;2019: 5403761. [74] LUCHAN J, CHOI C, CARRIER RL. Reactive oxygen species limit intestinal mucosa-bacteria homeostasis in vitro. Sci Rep. 2021;11(1):23727. [75] LAURIDSEN C. From oxidative stress to inflammation: redox balance and immune system. Poult Sci. 2019;98(10):4240-4246. [76] KUDRYAVTSEVA AV, KRASNOV GS, DMITRIEV AA, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016;7(29):44879-44905. [77] WANG Y, WU Y. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5):521. [78] FERRO D, BARATTA F, PASTORI D, et al. New insights into the pathogenesis of non-alcoholic fatty liver disease: gut-derived lipopolysaccharides and oxidative stress. Nutrients. 2020;12(9):2762. [79] WANG W, LI Q, CHAI W, et al. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Sci Nutr. 2019;7(8): 2636-2646. [80] MACH N, FUSTER-BOTELLA D. Endurance exercise and gut microbiota: a review. J Sport Health Sci. 2017;6(2):179-197. [81] QUEIPO-QRTUÑO MI, SEOANE LM, MURRI M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465. [82] ZHAO X, YI R, ZHOU X, et al. Preventive effect of Lactobacillus plantarum KSFY02 isolated from naturally fermented yogurt from Xinjiang, China, on d-galactose-induced oxidative aging in mice. J Dairy Sci. 2019;102(7):5899-5912. [83] SÁNCHEZ MACARRO M, VILA-GANDÍ AV, PÉREZ-PIÑERO S, et al. Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxidants (Basel). 2021;10(2):323. [84] WANG Y, FANG Z, ZHAI Q, et al. Supernatants of bifidobacterium longum and lactobacillusplantarum strains exhibited antioxidative effects on A7R5 cells. Microorganisms. 2021;9(2):452. [85] DALTON A, MERMIER C, ZUHL M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10(5):555-568. [86] WANG B, JIN YX, DONG JL, et al. Low-intensity exercise modulates gut microbiota to fight against radiation-induced gut toxicity in mouse models. Front Cell Dev Biol. 2021;9:706755. [87] LAMBERT JE, MYSLICKI JP, BOMHOF MR, et al. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab. 2015;40(7):749-752. [88] LAI Z, SHAN W, LI J, et al. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol Psychiatry. 2021;26(12): 7167-7187. [89] YANG W, LIU Y, YANG G, et al. Moderate-intensity physical exercise affects the exercise performance and gut microbiota of mice. Front Cell Infect Microbiol. 2021;11:712381. [90] WANG K, CAO G, ZHANG H, et al. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019;10(12):7844-7854. [91] REN J, GUO B, SUI H, et al. The effects of aerobic exercise on the intestinal tumors and flora of the ApcMin/+ mouse. Clin Transl Oncol. 2022;24(2):305-318. [92] LIU Z, LIU HY, ZHOU H, et al. Moderate-intensity exercise affects gut microbiome composition and influences cardiac function in myocardial infarction mice. Front Microbiol. 2017;8:1687. [93] WANG J, ZHANG Q, XIA J, et al. Moderate treadmill exercise modulates gut microbiota and improves intestinal barrier in high-fat-diet-induced obese mice via the ampk/cdx2 signaling pathway. Diabetes Metab Syndr Obes. 2022;15:209-223. [94] PETRIZ BA, CASTRO AP, ALMEIDA JA, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15(1):511. [95] YUAN X, XU S, HUANG H, et al. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand J Med Sci Sports. 2018;28(5):1541-1551. [96] DENOU E, MARCINKO K, SURETTE MG, et al. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310(11):E982-E993. [97] BARTON W, PENNEY NC, CRONIN O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625-633. |
| [1] | Fang Xingyan, Tian Zhenli, Zhao Zheyi, Wen Ping, Xie Tingting. Effects of sodium arsenite on human umbilical vein endothelial cell injury and sphingosine kinases 1/sphingosine 1-phosphate signaling axis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-7. |
| [2] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [3] | Tang Liang, Li Xiheng, Niu Ruijuan, Li Xinyue, Zou Xinying, Mao Tianjiao, Li Jiang. Naringin regulates the function of RAW264.7 macrophages to affect the osteogenic differentiation of MC-3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1205-1210. |
| [4] | Huang Linke, Wei Linhua, Jiang Jie, Liu Qian, Chen Weiwei. Effects of estrogen combined with treadmill exercise on bone mass and articular cartilage in ovariectomized mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1166-1171. |
| [5] | Ruan Ling, Wang Guanghua, Wu Rongping, Jin Zhan, Lyu Zhenqing, Zhang Nan, Li Shoubang. Correlation between exercise intensity and lipid metabolism disorder and oxidative stress in a high-diet rat model [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1149-1155. |
| [6] | Zhang Yan, He Ruibo, Wang Qingbo, Pi Yihua, Lu Chunmin, Xu Chuanyi, Ma Gang, Peng Peng. Effects of aerobic exercises with different load volumes on inflammatory response and insulin signaling pathway of skeletal muscle in obese rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1237-1244. |
| [7] | Wu Dongzhe, Gao Xiaolin, Li Chuangtao, Wang Hao. Constructing the prediction model of maximal oxygen uptake by back-propagation neural network based on the cardiorespiratory optimal point [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1224-1231. |
| [8] | Wang Ji, Zhang Min, Yang Zhongya, Zhang Long. A review of physical activity intervention in type 2 diabetes mellitus with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1272-1277. |
| [9] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [10] | Li Zhichao, Tan Guoqing, Su Hui, Xu Zhanwang, Xue Haipeng. Regulatory role of non-coding RNAs as potential therapeutic targets in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 758-764. |
| [11] | Wang Xiaoge, Liu Jiwen, Yang Shuai, Bao Jinyu, Li Cui. Effects of exercise on depression-like behaviors in chronic unpredictable mild stress rodent models: a systematic review and Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 813-820. |
| [12] | Wang Jinling, Huang Xiarong, Qu Mengjian, Huang Fujin, Yin Lingwei, Zhong Peirui, Liu Jin, Sun Guanghua, Liao Yang, Zhou Jun. Effects of exercise training on bone mass and bone microstructure in aged osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 676-682. |
| [13] | Li Yujiao, Su Kunxia. High-intensity endurance exercise influences browning of white adipose tissue in a mouse model of high-fat diet induced obesity [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 707-713. |
| [14] | Zhang Luyao, Yang Kang. Effects of different exercises on renal interstitial fibrosis in type 2 diabetic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 200-207. |
| [15] | Wu Shuangshuang, Zhang Ying, Kou Xianjuan. Treadmill exercise improves cognitive dysfunction in diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(2): 208-215. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||