中国组织工程研究 ›› 2012, Vol. 16 ›› Issue (49): 9201-9208.doi: 10.3969/j.issn.2095-4344.2012.49.015
• 干细胞移植 stem cell transplantation • 上一篇 下一篇
MRI活体示踪超顺磁性氧化铁标记兔骨髓间充质干细胞的自体皮下移植
金旭红1,张 寿1,杨 柳2,文亚明2,段小军2
- 1中南大学湘雅医学院附属海口市中心医院骨科,海南省海口市 570208;2解放军第三军医大学西南医院关节外科中心,重庆市 400038
-
收稿日期:2011-02-14修回日期:2012-03-27出版日期:2012-12-02发布日期:2013-01-16 -
通讯作者:张寿,主任医师,教授,硕士生导师,中南大学湘雅医学院附属海口市中心医院骨科,海南省海口市 570208 -
作者简介:金旭红,副主任医师,硕士生导师,主要从事骨科方面的研究。 -
基金资助:海口市重点科技计划项目(2010-168),应用自体骨髓间充质干细胞微创修复关节软骨缺损;(2009-049-11)利用脐血间充质干细胞构建生物工程软骨;海南省自然科学基金项目(309107),应用同种异体脐血干细胞构建生物工程软骨修复软骨缺损的实验研究。
In vivo magnetic resonance imaging tracking of superparamagnetic iron oxide labeled rabbit bone marrow mesenchymal stem cells after subcutaneoustransplantation
Jin Xu-hong1, Zhang Shou1, Yang Liu 2, Wen Ya-ming2, Duan Xiao-jun2
- 1Department of Orthopedics, Haikou Hospital Affiliated to Xiangya School of Medicine, Central South University, Haikou 570208, Hainan Province, China; 2Center of Joint Surgery, Southwest Hospital, Third Military Medical University, Chongqing 400038, China
-
Received:2011-02-14Revised:2012-03-27Online:2012-12-02Published:2013-01-16 -
About author:Jin Xu-hong, Associate chief physician, Master’s supervisor, Department of Orthopedics, Haikou Hospital Affiliated to Xiangya School of Medicine, Central South University, Haikou 570208, Hainan Province, China jxh53@yahoo.cn -
Supported by:Key Science and Technology Planning Program of Haikou, No. 2010-168*, 2009-049-11*; Natural Science Foundation of Haikou, No. 309107*
摘要:
背景:高磁场MRI已被成功应用于示踪移植超顺磁性氧化铁标记骨髓基质干细胞的研究,但目前还有应用低磁场MRI示踪移植干细胞的报道。 目的:探讨用0.2 T MRI活体示踪自体皮下移植磁化标记骨髓基质干细胞的分布和迁移的可行性。 方法:从兔骨髓中分离培养骨髓基质干细胞,采用超顺磁性氧化铁和BrdU双重标记后,与壳聚糖复合植入兔自体大腿皮下。自体皮下移植未标记骨髓基质干细胞和皮下单纯注射超顺磁性氧化铁为对照。 结果与结论:超顺磁性氧化铁标记骨髓基质干细胞经普鲁士蓝染色和电镜检查证实细胞胞浆含致密铁颗粒。超顺磁性氧化铁标记后自体皮下移植的兔骨髓基质干细胞在GRET2*WI序列成像时产生特征性的低信号改变至少维持8周,且信号逐渐从移植部位进入组织深处。但普鲁士蓝染色和BrdU免疫组化显示大部分的移植细胞仍停留在原移植部位。提示体外超顺磁性氧化铁能有效地标记骨髓基质干细胞,利用0.2 T MRI活体示踪自体皮下移植的超顺磁性氧化铁标记兔骨髓基质干细胞分布和迁移是可行的。
中图分类号:
引用本文
金旭红,张 寿,杨 柳,文亚明,段小军. MRI活体示踪超顺磁性氧化铁标记兔骨髓间充质干细胞的自体皮下移植[J]. 中国组织工程研究, 2012, 16(49): 9201-9208.
Jin Xu-hong, Zhang Shou, Yang Liu,Wen Ya-ming, Duan Xiao-jun. In vivo magnetic resonance imaging tracking of superparamagnetic iron oxide labeled rabbit bone marrow mesenchymal stem cells after subcutaneoustransplantation[J]. Chinese Journal of Tissue Engineering Research, 2012, 16(49): 9201-9208.
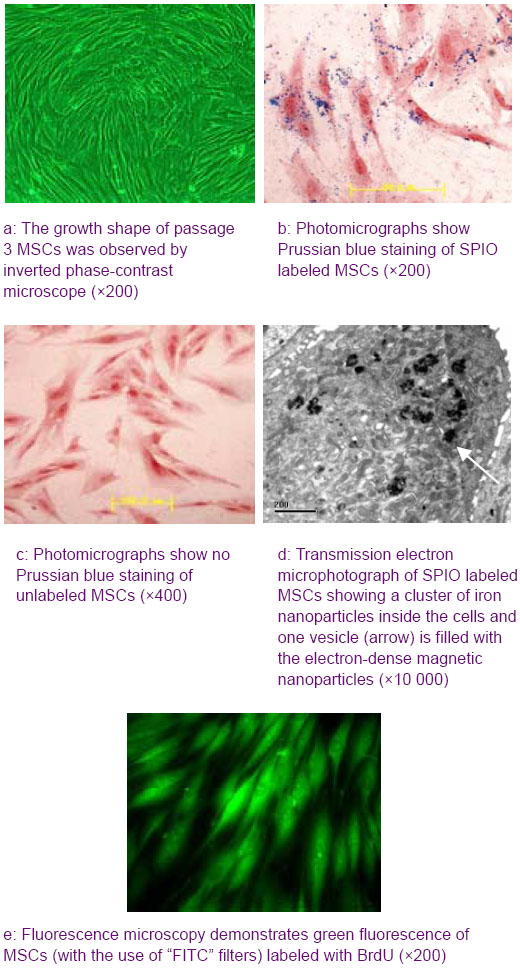
Quantitative analysis of experimental animals
Clear hypointense signals in the labeled MSCs and increasing numbers of hypointense regions with increasing concentrations of labeled MSCs are shown in Figure 2. GRET2*WI sequence demonstrated significant decreases in the signal intensity for 1×106 and 5×105 labeled cells compared with unlabeled cells, but 1×106 labeled cells had the greater loss of signal
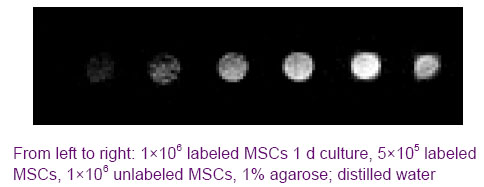
At 5 days post-grafting in group A animals, a lonely significant hypointense signal void located in the subcutaneous site can be identified, which was detected at a distance of 0.5 cm from implantation site on axial MR images (Figure 3c), the maximum diameter of hypointense signal void with irregular
borders dispersing to 1.7 cm can be identified on sagittal MR images, and there was no decreases in the signal intensity. The hypointense signal void was disappeared after 5 days post-grafting in group C animals, and no contrast could be observed on MR images of unlabeled cells in B group animals. At 2 weeks post-grafting in group A, the lonely significant hypointense signal void was observed connecting with marked hypointense signal caused by the injection site, and appeared as a dark elongated line long as 0.6 cm on axial MR images (Figure 3d), the maximum diameter of hypointense signal area dispersing to 2.0 cm on sagittal MR images, and it had involved in the muscle layer. Over the following weeks, we observed the hypointense signal line was intensified and elongated, and the size of hypointense signal area was increased. At 8 weeks post-grafting, the hypointense signal line reached its maximum of 1.1 cm (Figure 3e), and the maximum diameter of hypointense signal area which encroached in the muscle deep layer was observed spreading to 2.6 cm. But a decreasing in hypointense signal intensity was seen (Figure 3f). Figure 3 Transverse and saggital sections T2*-weighted gradient echo images from rabbit hind limb after autologous implantation of labeled mesenchymal stem cells in subcutaneous tissue

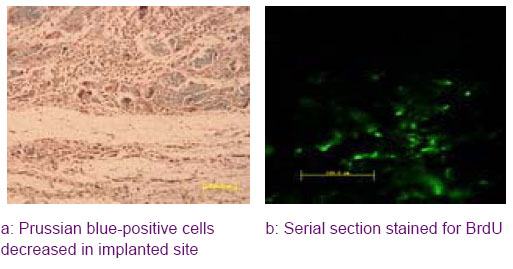
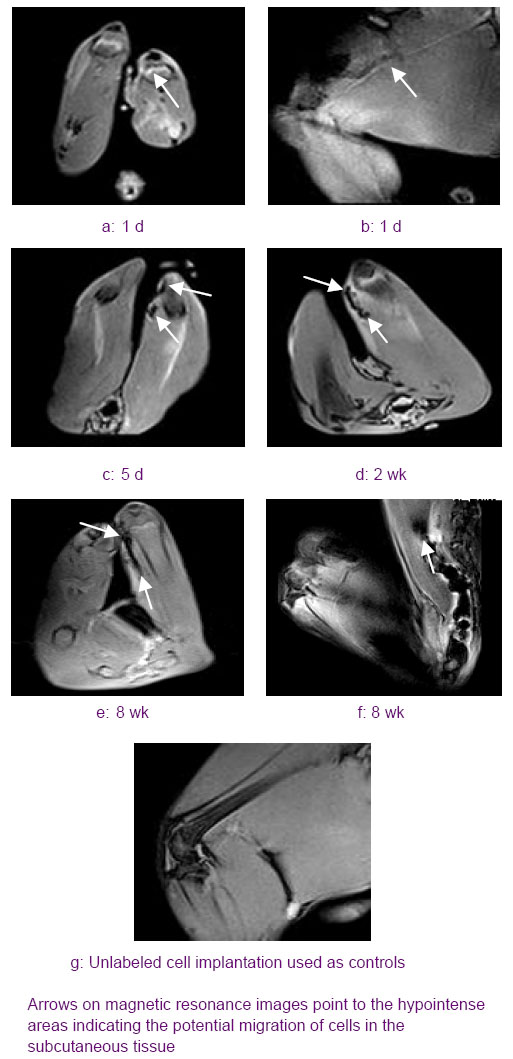
Histological and immunohistochemical evaluation of the implanted MSCs
| [1] Kuroda R, Ishida K, Matsumoto T, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15(2):226-231. [2] Zhou G, Liu W, Cui L, et al. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12(11):3209-3221. [3] Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614-2623. [4] Chalfie M, Tu Y, Euskirchen G, et al. Green fluorescent protein as a marker for gene expression. Science. 1994; 263(5148):802-805. [5] Duan X, Yang L, Dong S, et al. Characterization of EGFP-labeled mesenchymal stem cells and redistribution of allogeneic cells after subcutaneous implantation. Arch Orthop Trauma Surg. 2008;128(7):751-759. [6] Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1): 54-63.[7] Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1): 9-20. [8] Rüster B, Göttig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938-3944.[9] Runnels JM, Zamiri P, Spencer JA, et al. Imaging molecular expression on vascular endothelial cells by in vivo immunofluorescence microscopy. Mol Imaging. 2006;5(1): 31-40. [10] Yoon YS, Park JS, Tkebuchava T, et al. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109(25): 3154-3157. [11] Rochefort GY, Vaudin P, Bonnet N, et al. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies? Respir Res. 2005;6:125.[12] Arbab AS, Bashaw LA, Miller BR, et al. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation. 2003;76(7):1123-1130. [13] Farrell E, Wielopolski P, Pavljasevic P, et al. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem Biophys Res Commun. 2008;369(4):1076-1081.[14] Elgort DR, Duerk JL. A review of technical advances in interventional magnetic resonance imaging. Acad Radiol. 2005;12(9):1089-1099. [15] Ejbjerg BJ, Narvestad E, Jacobsen S, et al. Optimised, low cost, low field dedicated extremity MRI is highly specific and sensitive for synovitis and bone erosions in rheumatoid arthritis wrist and finger joints: comparison with conventional high field MRI and radiography. Ann Rheum Dis. 2005;64(9):1280-1287. [16] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.[17] Jing XH, Yang L, Duan XJ, et al. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Joint Bone Spine. 2008;75(4):432-438.[18] Arbab AS, Yocum GT, Kalish H, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104(4):1217-1223. [19] Zhang Z, van den Bos EJ, Wielopolski PA, et al. In vitro imaging of single living human umbilical vein endothelial cells with a clinical 3.0-T MRI scanner. Magma. 2005;18(4):175-185. [20] Hsieh CH, Lee MC, Tsai-Wu JJ, et al. Deleterious effects of MRI on chondrocytes. Osteoarthritis Cartilage. 2008;16(3): 343-351. [21] Tallheden T, Nannmark U, Lorentzon M, et al. In vivo MR imaging of magnetically labeled human embryonic stem cells. Life Sci. 2006;79(10):999-1006.[22] Østergaard M, Ejbjerg B, Szkudlarek M. Imaging in early rheumatoid arthritis: roles of magnetic resonance imaging, ultrasonography, conventional radiography and computed tomography. Best Pract Res Clin Rheumatol. 2005;19(1): 91-116. [23] Schirmer C, Scheel AK, Althoff CE, et al. Diagnostic quality and scoring of synovitis, tenosynovitis and erosions in low-field MRI of patients with rheumatoid arthritis: a comparison with conventional MRI. Ann Rheum Dis. 2007; 66(4):522-559.[24] Arbab AS, Bashaw LA, Miller BR, et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229(3):838-846.[25] Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228(2):480-487.[26] Suh JS, Lee JY, Choi YS, et al. Efficient labeling of mesenchymal stem cells using cell permeable magnetic nanoparticles. Biochem Biophys Res Commun. 2009; 379(3):669-675. [27] Farrell E, Wielopolski P, Pavljasevic P, et al. Cell labelling with superparamagnetic iron oxide has no effect on chondrocyte behaviour. Osteoarthritis Cartilage. 2009;17(7):961-967. [28] Ju S, Teng G, Zhang Y, et al. In vitro labeling and MRI of mesenchymal stem cells from human umbilical cord blood. Magn Reson Imaging. 2006;24(5):611-617. [29] Bulte JWM. Magnetic nanoparticles as markers for cellular MR imaging. J Magnetism Magnetic Materials. 2005;289: 423-427.[30] Arbab AS, Pandit SD, Anderson SA, et al. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells. 2006;24(3):671- 678. [31] George AJT, Bhakoo KK, Haskard, DO, et al. Imaging Molecular and Cellular Events in Implantation. Implantation. 2006;82:1124-1129.[32] Magnitsky S, Watson DJ, Walton RM, et al. In vivo and ex vivo MRI detection of localized and disseminated neural stem cell grafts in the mouse brain. Neuroimage. 2005; 26(3):744-754.[33] Ding W, Bai J, Zhang J, et al. In vivo tracking of implanted stem cells using radio-labeled transferrin scintigraphy. Nucl Med Biol. 2004;31(6):719-725. [34] Bulte JW, Zhang S, van Gelderen P, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci U S A. 1999;96(26):15256-15261. [35] Jendelová P, Herynek V, DeCroos J, et al. Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn Reson Med. 2003;50(4):767-776. |
| [1] | 刘 洋, 刘东辉, 徐 磊, 展 旭, 孙昊博, 康 凯. 刺激响应型可注射水凝胶在心肌梗死精准化治疗中的作用与趋势[J]. 中国组织工程研究, 2026, 30(8): 2072-2080. |
| [2] | 赖 渝, 陈跃平, 章晓云. 生物活性材料治疗骨感染的研究热点与前沿趋势[J]. 中国组织工程研究, 2026, 30(8): 2132-2144. |
| [3] | 王奇飒, 卢雨征, 韩秀峰, 赵文玲, 石海涛, 徐 哲. 3D打印甲基丙烯酰化透明质酸/脱细胞皮肤水凝胶支架的细胞相容性[J]. 中国组织工程研究, 2026, 30(8): 1912-1920. |
| [4] | 王菘芃, 刘玉三, 于焕英, 高晓丽, 徐英江, 张晓明, 刘 敏. 沸石基咪唑盐框架8纳米材料的活性氧双向调控:从肿瘤治疗、抗菌到细胞保护[J]. 中国组织工程研究, 2026, 30(8): 2033-2013. |
| [5] | 王明琦, 冯诗雅, 韩银河, 于朋鑫, 郭丽娜, 贾子萱, 王秀丽. 神经化肠黏膜组织工程模型的构建及体外评价[J]. 中国组织工程研究, 2026, 30(4): 892-900. |
| [6] | 余诗宇, 俞苏桐, 徐 杨, 镇祥燕, 韩凤选. 组织工程治疗策略在口腔黏膜下纤维化中的研究与应用进展[J]. 中国组织工程研究, 2026, 30(4): 936-948. |
| [7] | 杨 虎, 郑 宇, 贾承明, 王 通, 张广飞, 纪垚垚. 免疫微环境调节骨再生[J]. 中国组织工程研究, 2026, 30(3): 701-710. |
| [8] | 闫启全, 杨立斌, 李梦君, 倪亚卓, 陈科颖, 许 博, 李耀扬, 马士卿, 李 睿, 李建文. 负载抗菌肽KR-12-a5猪小肠黏膜下层复合纳米羟基磷灰石生物支架的制备及抗菌性能[J]. 中国组织工程研究, 2026, 30(2): 384-394. |
| [9] | 袁 茜, 张 昊, 庞 杰. 负载柚皮苷壳聚糖/β-磷酸三钙支架的表征及生物学性能[J]. 中国组织工程研究, 2026, 30(2): 424-432. |
| [10] | 王 卓, 孙盼盼, 程焕芝, 曹婷婷. 壳聚糖在口腔软硬组织修复与再生中的应用[J]. 中国组织工程研究, 2026, 30(2): 459-468. |
| [11] | 王 域, 范民杰, 郑朋飞. 多重刺激响应性水凝胶在骨损伤修复中的应用:特殊响应能力及多样性功能[J]. 中国组织工程研究, 2026, 30(2): 469-479. |
| [12] | 顾健美, 袁坤山, 周 强, 张海军, . 激光微孔化脱细胞支架在组织再生中的应用[J]. 中国组织工程研究, 2026, 30(2): 499-507. |
| [13] | 杨凤丽, 周 朝, 熊 伟, 周宇翔, 李登顺, 王 鑫, 李展振. 3D打印聚乳酸骨支架修复骨缺损[J]. 中国组织工程研究, 2026, 30(2): 507-515. |
| [14] | 姜 侃, 阿力木江·阿不都肉苏力, 沙拉依丁·艾尔西丁, 艾克拜尔江·艾赛提, 库提鲁克·守克尔, 艾克热木江·木合热木. 生物材料与骨再生:研究热点及有影响力的500篇文献分析[J]. 中国组织工程研究, 2026, 30(2): 528-536. |
| [15] | 薛 慧, 李东楠, 赵雅迪, 陈 超, 谢宗源. Ph染色体阳性急性淋巴细胞白血病移植前后BCR/ABL基因表达与复发的关系[J]. 中国组织工程研究, 2026, 30(1): 139-144. |
Design
A randomized controlled animal experiment.
Time and setting
Materials
A total of 14 male Japanese White rabbits, aged 8-week-old and weighting 2-2.5 kg, were provided by the Experimental Animal Center of the Third Military Medical University in China. The protocols employed were in direct accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[16].
.jpg)
Isolation and culture of MSCs
SPIO labeling of cells
Iron detection
In vitro proliferation of MSCs
In vitro cellular MRI
Experimental protocol
In vivo experiments and in vivo MRI
Histology and immunohistochemistry
Main outcome measures
A low-field dedicated extremity MRI unit (0.2 T) was applied to detect the SPIO-labeled MSCs following subcutaneous implantation in vivo at different time points in rabbit models.
兔骨髓基质干细胞采用超顺磁性氧化铁和BrdU双重标记后,与壳聚糖复合植入兔自体大腿皮下,利用0.2T MRI可以活体示踪自体皮下移植的超顺磁性氧化铁标记兔骨髓基质干细胞分布和迁移。
超顺磁氧化铁静脉给药后,由于颗粒小于血细胞,可通过肺、脑、心、肾的血管床,而后被枯否细胞吞噬分布干全身的网状内皮系统,其中肝脏的枯否细胞可吞噬给药量的80%,并被代谢成可被红细胞和血红蛋白利用的铁离子。肝脾MRI信号恢复正常需3~7d。肝细胞增强后显示低黑信号,无枯否细胞的病变组织如癌组织信号不改变,从而形成明显的对比反差。肝肿瘤大于3min即可检出。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||