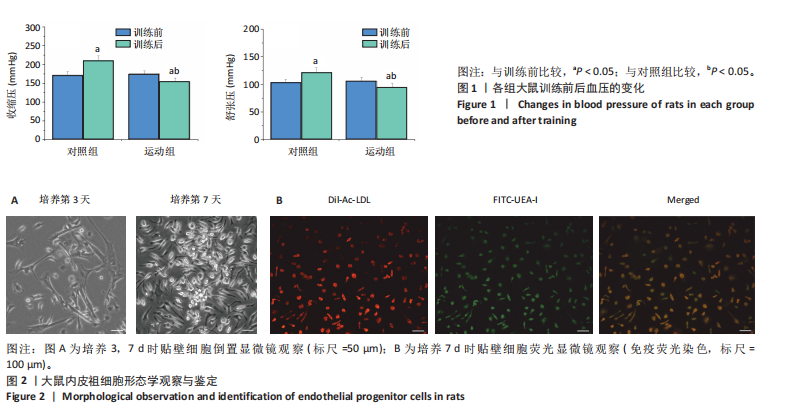
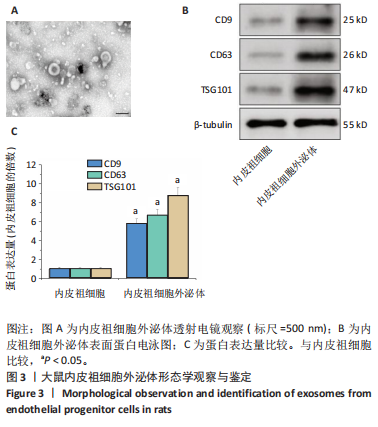
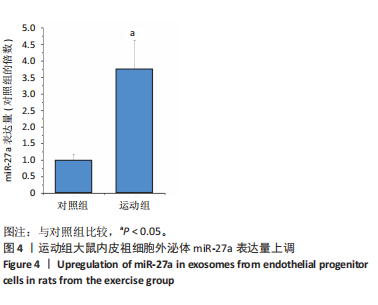
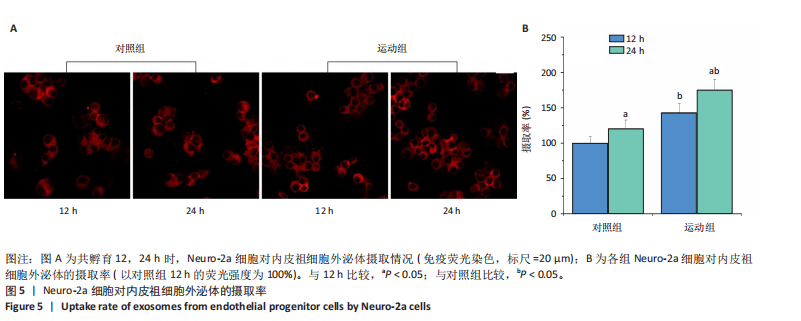
[1] POTTER T, TANNOUS J, VAHIDY FS. A Contemporary review of epidemiology, risk factors, etiology, and outcomes of premature stroke. Curr Atheroscler Rep. 2022;24(12):939-948.
[2] MALIK AN, TARIQ H, AFRIDI A, et al. Technological advancements in stroke rehabilitation. J Pak Med Assoc. 2022;72(8):1672-1674.
[3] KIM Y, SHARP S, HWANG S, et al. Exercise and incidence of myocardial infarction, stroke, hypertension, type 2 diabetes and site-specific cancers: prospective cohort study of 257 854 adults in South Korea. BMJ Open. 2019;9(3):e025590.
[4] BAKKER EA, SUI X, BRELLENTHIN AG, et al. Physical activity and fitness for the prevention of hypertension. Curr Opin Cardiol. 2018;33(4): 394-401.
[5] XIA X, LI G, DONG Q, et al. Endothelial progenitor cells as an emerging cardiovascular risk factor in the field of food and nutrition research: advances and challenges. Crit Rev Food Sci Nutr. 2024;64(33):12166-12183.
[6] KING TF, MCDERMOTT JH. Endothelial progenitor cells and cardiovascular disease. J Stem Cells. 2014;9(2):93-106.
[7] LUO S, XIA W, CHEN C, et al. Endothelial progenitor cells and hypertension: current concepts and future implications. Clin Sci (Lond). 2016;130(22):2029-2042.
[8] MARKETOU ME, KALYVA A, PARTHENAKIS FI, et al. Circulating endothelial progenitor cells in hypertensive patients with increased arterial stiffness. J Clin Hypertens (Greenwich). 2014;16(4):295-300.
[9] CAVALCANTE SL, LOPES S, BOHN L, et al. Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Rev Port Cardiol (Engl Ed). 2019;38(11):817-827.
[10] FERENTINOS P, TSAKIRIDES C, SWAINSON M, et al. The impact of different forms of exercise on circulating endothelial progenitor cells in cardiovascular and metabolic disease. Eur J Appl Physiol. 2022;122(4): 815-860.
[11] MITSIOU G, TOKMAKIDIS SP, DINAS PC, et al. Endothelial progenitor cell mobilization based on exercise volume in patients with cardiovascular disease and healthy individuals: a systematic review and meta-analysis. Eur Heart J Open. 2022;2(6):e078.
[12] DE SOUZA MESQUITA FO, GAMBASSI BB, DE OLIVEIRA SILVA M, et al. Effect of high-intensity interval training on exercise capacity, blood pressure, and autonomic responses in patients with hypertension: A systematic review and meta-analysis. Sports Health. 2023;15(4):571-578.
[13] KRYLOVA SV, FENG D. The machinery of exosomes: Biogenesis, release, and uptake. Int J Mol Sci. 2023;24(2):e1337.
[14] KIMIZ-GEBOLOGLU I, ONCEL SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533-543.
[15] CHEN S, POLAKI V, BIHL JC, et al. Compromised endothelial progenitor cell exosomal communication with endothelial cells in hypertension ischemia conditions. Front Stroke. 2022;1:e1015463.
[16] TE RIET L, VAN ESCH JH, ROKS AJ, et al. Hypertension: renin-angiotensin- aldosterone system alterations. Circ Res. 2015;116(6):960-975.
[17] CHEN S, LI G, ZHANG W, et al. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1526-1531.
[18] NI P, YANG L, LI F. Exercise-derived skeletal myogenic exosomes as mediators of intercellular crosstalk: a major player in health, disease, and exercise. J Physiol Biochem. 2023;79(3):501-510.
[19] FRüHBEIS C, HELMIG S, TUG S, et al. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:e28239.
[20] CHATURVEDI P, KALANI A, MEDINA I, et al. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med. 2015;19(9):2153-2161.
[21] MA C, WANG J, LIU H, et al. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med Sci Sports Exerc. 2018;50(10):2024-2032.
[22] YOON KJ, PARK S, KWAK SH, et al. Effects of voluntary running wheel exercise-induced extracellular vesicles on anxiety. Front Mol Neurosci. 2021;14:665800.
[23] 袁国强,秦永生,彭朋.高强度间歇运动对自发性高血压模型大鼠病理性心脏肥大的影响及机制[J].中国组织工程研究,2020, 24(23):3708-3715.
[24] ERKEN HA, ERKEN G, GENÇ O. Blood pressure measurement in freely moving rats by the tail cuff method. Clin Exp Hypertens. 2013;35(1): 11-15.
[25] 卫肖艳,张莉,林明,等.大鼠骨髓源性内皮祖细胞的分离培养与鉴定[J].中国组织工程研究,2013,17(14):2570-2577.
[26] ZHAO JL, ZHAO L, ZHAN QN, et al. BMSC-derived exosomes ameliorate peritoneal dialysis-associated peritoneal fibrosis via the miR-27a-3p/TP53 Pathway. Curr Med Sci. 2024;44(2):333-345.
[27] ZHANG C, WANG J, MA X, et al. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J Cell Mol Med. 2018;22(3):1873-1882.
[28] 彭阿建,欧阳范馨,张熙,等.黄芪甲苷干预的EPC-Exos对高糖诱导损伤间充质干细胞向内皮分化的影响[J].世界科学技术-中医药现代化,2022,24(4):1593-1602.
[29] KHATTAR KE, SAFI J, RODRIGUEZ AM, et al. Intercellular communication in the brain through tunneling nanotubes. Cancers (Basel). 2022;14(5): e1207.
[30] MARAR C, STARICH B, WIRTZ D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5): 560-570.
[31] LI J, MA Y, MIAO XH, et al. Neovascularization and tissue regeneration by endothelial progenitor cells in ischemic stroke. Neurol Sci. 2021; 42(9):3585-3593.
[32] WANG J, LIU H, CHEN S, et al. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Exp Neurol. 2020;330: e113325.
[33] CORREIA RR, BATISTA V, VERAS A, et al. High-intensity interval training attenuates the effects caused by arterial hypertension in the ventral prostate. Prostate. 2022;82(3):373-387.
[34] 付常喜,马刚,李平,等.不同强度运动对自发性高血压大鼠肾脏纤维化的影响及作用机制研究[J].首都体育学院学报,2021, 33(6):638-648.
[35] 张敏,彭朋,秦永生,等.不同负荷剂量高强度间歇训练对高血压肾病大鼠肾脏损伤的影响[J].山东体育学院学报,2020,36(6):54-64.
[36] 白春宏,秦永生,薄海,等.不同运动方式对自发性高血压大鼠骨骼肌纤维类型与代谢的影响[J].中国康复医学杂志,2020,35(3): 294-300.
[37] ZHENG D, HUO M, LI B, et al. The role of exosomes and exosomal microRNA in cardiovascular disease. Front Cell Dev Biol. 2020;8: e616161.
[38] DARRAGH I, O’DRISCOLL L, EGAN B. Exercise training and circulating small extracellular vesicles: Appraisal of methodological approaches and current knowledge. Front Physiol. 2021;12:e738333.
[39] CASTAñO C, MIRASIERRA M, VALLEJO M, et al. Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc Natl Acad Sci U S A. 2020;117(48):30335-30343.
[40] XI T, JIN F, ZHU Y, et al. MiR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J Biol Chem. 2018;293(52):20041-20050.
[41] CHEN Q, XU J, LI L, et al. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 2014;5(3):e1132.
[42] GU Q, WANG B, ZHANG XF, et al. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc Pathol. 2014;23(5):298-305.
[43] HERGENHAHN L, PADUTSCH N, AZAWI S, et al. Cytogenomic characterization of murine neuroblastoma cell line Neuro-2a and its two derivatives Neuro-2a TR-alpha and Neuro-2a TR-beta. Cells. 2024;13(22):e1889.
[44] XIA C, DAI Z, JIN Y, et al. Emerging antioxidant paradigm of mesenchymal stem cell-derived exosome therapy. Front Endocrinol (Lausanne). 2021;12:727-739.
[45] ŞIŞLI HB, HAYAL TB, ŞENKAL S, et al. Apelin receptor signaling protects GT1-7 GnRH neurons against oxidative stress in vitro. Cell Mol Neurobiol. 2022;42(3):753-775.
[46] DU ZD, YU S, QI Y, et al. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging model in rats. Neurochem Int. 2019;124:31-40.
[47] JUN Z, LEI W, CE F, et al. NADPH oxidase 4 facilitates progression of chondrosarcoma via generation of reactive oxygen species. Acta Biochim Pol. 2023;70(3):685-692.
|