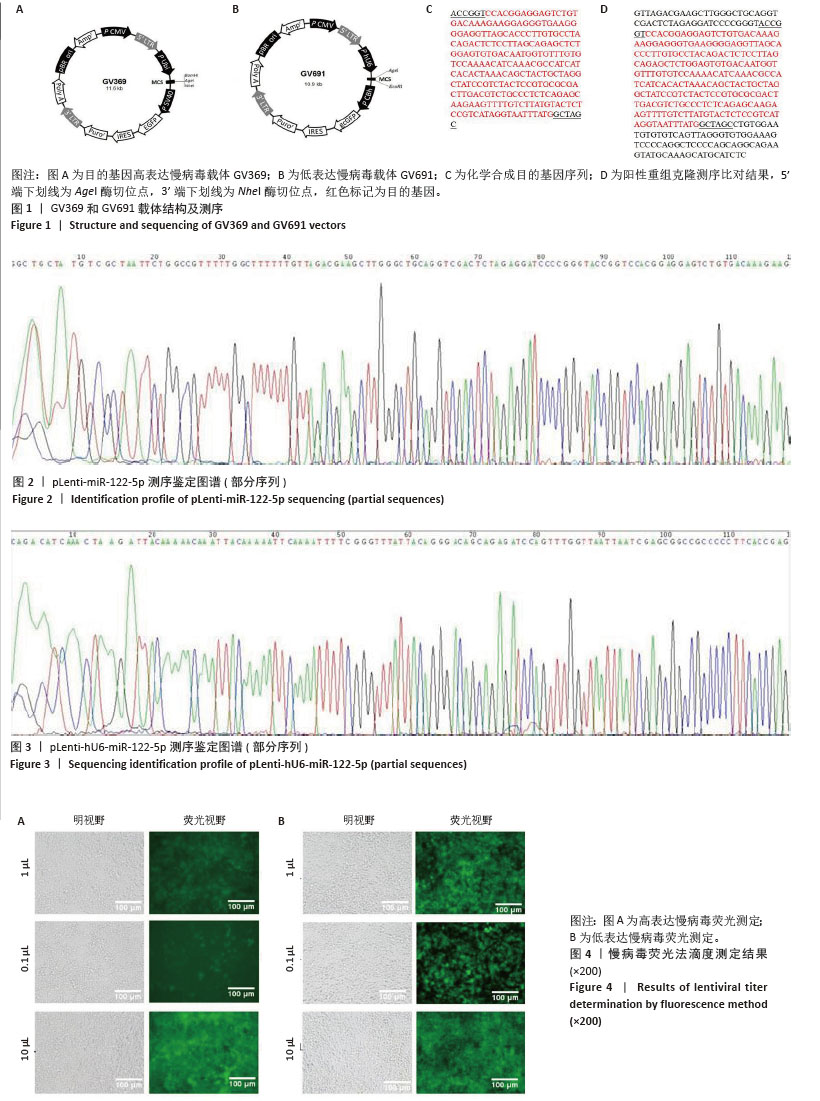
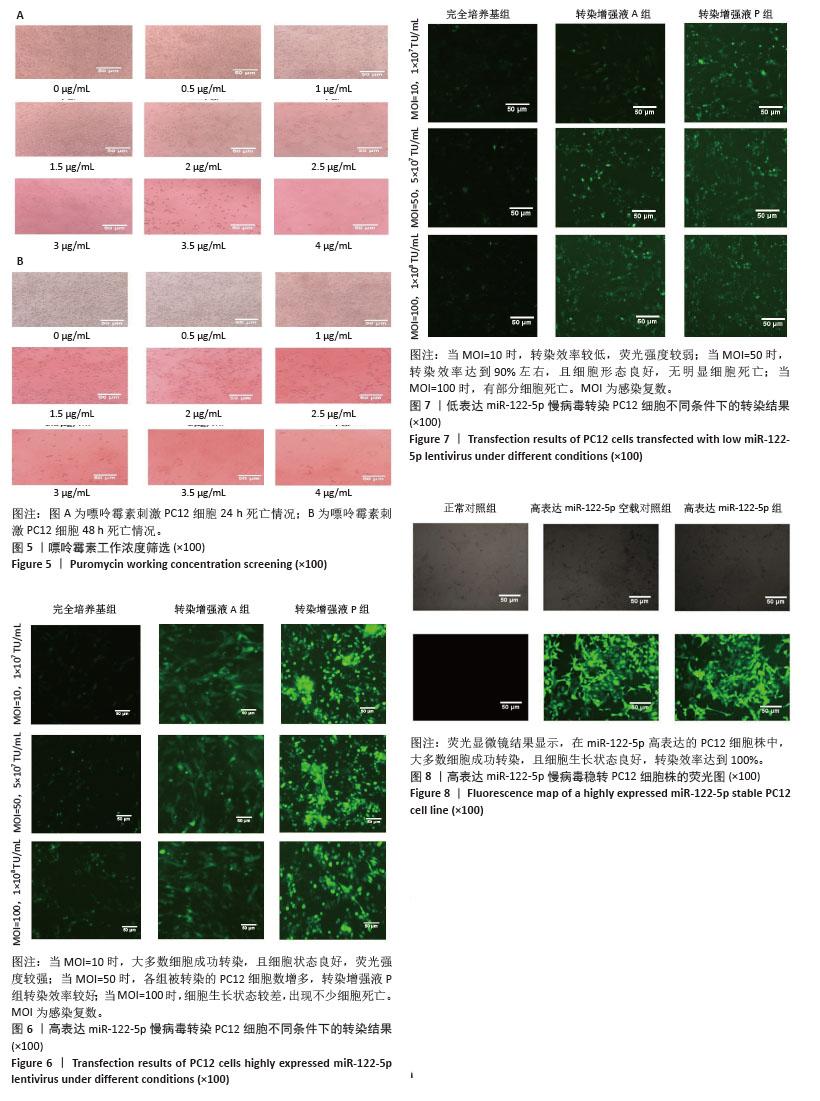
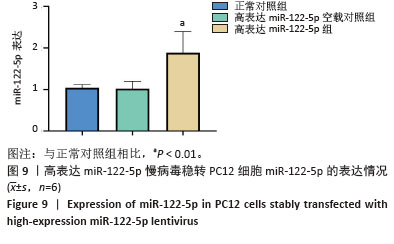
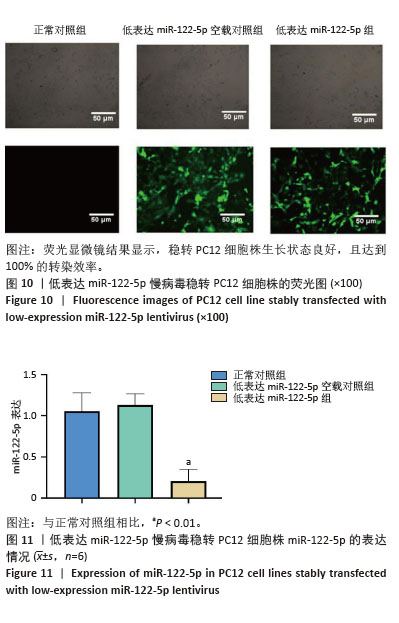
[1] FU G, BRKIĆ J, HAYDER H, et al. MicroRNAs in Human Placental Development and Pregnancy Complications. Int J Mol Sci. 2013;14(3): 5519-5544.
[2] CHO JR, LEE CY, LEE J, et al. MicroRNA-761 inhibits Angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin.Clin Hemorheol Micro. 2015;63(1):45-56.
[3] LIN J, ZHAN R. [Advance of studies on role of miRNA in hematopoietic regulation and myeloproliferative neoplasms] Chin Assoc Pathophysiol. 2011;19:1071-1074.
[4] LEE Y, AHN C, HAN J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415-419.
[5] BARTEL DP. Metazoan MicroRNAs. Cell. 2018;173(1):20-51.
[6] SAIYED AN, VASAVADA AR, JOHAR SRK. Recent trends in miRNA therapeutics and the application of plant miRNA for prevention and treatment of human diseases.Futur J Pharm Sci. 2022;8(1):24.
[7] CHIPMAN LB, PASQUINELLI AE. miRNA Targeting: Growing beyond the Seed. Trends Genet. 2019;35(3):215-222.
[8] AZIMI M, TOTONCHI M, EBRAHIMI M. Determining The Role of MicroRNAs in Self-Renewal, Metastasis and Resistance to Drugs in Human Gastric Cancer Based on Data Mining Approaches: A Systematic Review. Cell J. 2022;24(1):1-6.
[9] GOLHANI V, RAY SK, MUKHERJEE S. Role of MicroRNAs and Long Non-Coding RNAs in Regulating Angiogenesis in Human Breast Cancer: A Molecular Medicine Perspective. Curr Mol Med. 2022;22(10):882-893.
[10] MA L, HUO Y, TANG Q, et al. Human Breast Milk Exosomal miRNAs are Influenced by Premature Delivery and Affect Neurodevelopment. Mol Nutr Food Res. 2024;68(9):e2300113.
[11] ASAKIYA C, ZHU L, YUHAN J, et al. Current progress of miRNA-derivative nucleotide drugs: modifications, delivery systems, applications. Expert Opin Drug Del. 2022;19(4):435-450.
[12] MAGED AM, DEEB WS, EL AMIR A, et al. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis.Int J Gynecol Obstet. 2018;141(1):14-19.
[13] ZHAN G, JIANG H, YANG R, et al. miR-122 and miR-197 expressions in hepatic carcinoma patients before and after chemotherapy and their effect on patient prognosis. Am J Transl Res. 2021;13(6):6731-6737.
[14] ZHANG K, XU X, HU L. Sevoflurane attenuates hepatic ischemia reperfusion injury by the miR-122/Nrf2 pathway.Ann Transl Med. 2022;10(6):350.
[15] WANG L, CHEN H. Correlation between serum miR-122 and myocardial damage and ventricular function in patients with essential hypertension. J Thorac Dis. 2021;13(8):4999-5006.
[16] JAMPOKA K, MUANGPAISARN P, KHONGNOMNAN K, et al. Serum miR-29a and miR-122 as Potential Biomarkers for Non-Alcoholic Fatty Liver Disease (NAFLD). Microrna. 2018;7(3):215-222.
[17] WU X, DU X, YANG Y, et al. Inhibition of miR-122 reduced atherosclerotic lesion formation by regulating NPAS3-mediated endothelial to mesenchymal transition.Life Sci. 2021;265:118816.
[18] CHEN Z, FU S, SHAN Y, et al. Circ_0001047 inhibits prostate cancer progression and enhances abiraterone sensitivity via miR-122-5p/FKBP5/PHLPP1/AKT axis in vitro. Discov Oncol. 2024;15(1):569.
[19] VOKACOVA K, LANDECKA A, SELVI S, et al. Plasma miR-122-5p and miR-142-5p and their role in chemoresistance of colon cancer patients. Mutagenesis. 2025;40(1):80-86.
[20] ZHANG J, LI K, GAO L, et al. Glucose metabolism disorder related to follicular fluid exosomal miR-122-5p in cumulus cells of endometriosis patients. Reproduction. 2024;168(4):e240028.
[21] LIU F, LIU B, XU S, et al. MicroRNA-122 protects against interferon-α-induced hepatic inflammatory response via the Janus kinase-signal transducer and activator of transcription pathway. Endocr J. 2025;72(1):53-67.
[22] SONG C, LI S, MAI Y, et al. Dysregulated expression of miR-140 and miR-122 compromised microglial chemotaxis and led to reduced restriction of AD pathology. J Neuroinflammation. 2024;21(1):167.
[23] LIN J, ZHENG X. Salvianolate Blocks Apoptosis During Myocardial Infarction by Down Regulating miR-122-5p. Curr Neurovasc Res. 2017; 14(4): 323-329.
[24] MANKHONG S, KIM S, MOON S, et al. Circulating micro-RNAs Differentially Expressed in Korean Alzheimer’s Patients With Brain Aβ Accumulation Activate Amyloidogenesis. J Gerontol A-Biol. 2023;78(2): 292-303.
[25] 王蒙蒙,王怡,林平. miR-122与急性缺血性脑卒中的相关性[J].中国老年学杂志,2022,42(5):1053-1055.
[26] 黄蔚文, 赵岳峰. 重型颅脑损伤患者外周血miR-122-5p表达水平变化及其对近期预后的预测价值[J].卒中与神经疾病,2021,28(1): 88-91.
[27] LEWIS BP, BURGE CB, BARTEL DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15-20.
[28] PENG Y, CROCE CM. The role of MicroRNAs in human cancer. Signal Transduct Tar. 2016;1:15004.
[29] CAN U, MARZIOGLU E, AKDU S. Some miRNA expressions and their targets in ischemic stroke. Nucleos Nucleot Nucl. 2022;41(11): 1224-1262.
[30] YANG F, NING Z, MA L, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16(1): 148.
[31] BAEK J, KANG S, MIN H. MicroRNA-targeting therapeutics for hepatitis C. Arch Pharm Res. 2014;37(3):299-305.
[32] KHOODORUTH MAS, KHOODORUTH WNC, UROOS M, et al. Diagnostic and mechanistic roles of MicroRNAs in neurodevelopmental & neurodegenerative disorders. Neurobiol Dis. 2024;202:106717.
[33] LIU T, WU H, LI J, et al. Unraveling the Bone-Brain Axis: A New Frontier in Parkinson’s Disease Research. Int J Mol Sci. 2024;25(23):12842.
[34] SAWANT H, SUN B, MCGRADY E, et al. Role of miRNAs in neurovascular injury and repair. J Cerebr Blood F Met. 2024;44(10):1693-1708.
[35] HINTERMAYER MA, JUŹWIK CA, MORQUETTE B, et al. A miR-383-5p Signaling Hub Coordinates the Axon Regeneration Response to Inflammation. J Neurosci. 2024;44(44):e1822232024.
[36] 郭占非,吴洁,杨学华.miR-122-5p对类风湿关节炎滑膜细胞增殖与凋亡的影响及其机制[J].青岛大学学报(医学版),2022,58(2): 289-293.
[37] VIBO R, JÕGI K, REMM A, et al. Different expression patterns of inflammation-related genes and serum microRNAs in young-onset ischemic stroke. Sci Rep. 2024;14(1):23845.
[38] 罗凯, 段华彬, 罗明鼎.瑞舒伐他汀抑制miR-122-5p减轻LPS诱导的神经细胞损伤[J].河北医药,2022,44(5):659-663.
[39] LV B, CHENG X, SHARP FR, et al. MicroRNA-122 Mimic Improves Stroke Outcomes and Indirectly Inhibits NOS2 After Middle Cerebral Artery Occlusion in Rats. Front Neurosci. 2018;12:767.
[40] GUO D, MA J, LI T, et al. Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-κB pathway by targeting FOXO3. Exp Cell Res. 2018; 369(1):34-42.
[41] MARTIN TF, GRISHANIN RN. PC12 cells as a model for studies of regulated secretion in neuronal and endocrine cells.Method Cell Biol. 2003;71: 267-286.
[42] XIE D, DENG T, ZHAI Z, et al. The cellular model for Alzheimer’s disease research: PC12 cells.Front Mol Neurosci. 2022;15:1016559.
[43] SAVENKOVA DA, MAKAROVA AA, SHALIK IK, et al. miRNA Pathway Alteration in Response to Non-Coding RNA Delivery in Viral Vector-Based Gene Therapy. Int J Mol Sci. 2022;23(23):14954.
[44] SKUKAN L, BREZAK M, ISTER R, et al. Lentivirus- or AAV-mediated gene therapy interventions in ischemic stroke: A systematic review of preclinical in vivo studies. J Cerebr Blood F Met. 2022;42(2):219-236. |