中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (8): 2044-2053.doi: 10.12307/2026.026
• 生物材料综述 biomaterial review • 上一篇 下一篇
抗氧化纳米材料在口腔中的应用和不足
杨学涛,朱梦菡,张宸熙,孙一民,叶 玲
- 口腔疾病防治全国重点实验室,国家口腔疾病临床医学研究中心,四川大学华西口腔医院牙体牙髓科,四川省成都市 610041
-
收稿日期:2024-11-25接受日期:2025-01-24出版日期:2026-03-18发布日期:2025-07-18 -
通讯作者:叶玲,博士,教授,口腔疾病防治全国重点实验室,国家口腔疾病临床医学研究中心,四川大学华西口腔医院牙体牙髓科,四川省成都市 610041 共同孙一民,博士,副教授,口腔疾病防治全国重点实验室,国家口腔疾病临床医学研究中心,四川大学华西口腔医院牙体牙髓科,四川省成都市 610041 -
作者简介:杨学涛,男,1999年生,四川省凉山州人,汉族,四川大学华西口腔医学院在读硕士,主要从事抗氧化对口腔的影响研究。 -
基金资助:国家自然科学基金项目(U21A20368),项目负责人:叶玲;中国博士后科学基金资助项目(BX20230240),项目负责人:孙一民;四川省科技计划项目(2024NSFSC1581),项目负责人:孙一民
Applications and limitations of antioxidant nanomaterials in oral cavity
Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling
- Department of Endodontics, National Key Laboratory of Oral Disease Prevention and Control & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2024-11-25Accepted:2025-01-24Online:2026-03-18Published:2025-07-18 -
Contact:Ye Ling, MD, Professor, Department of Endodontics, National Key Laboratory of Oral Disease Prevention and Control & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China Sun Yimin, MD, Associate professor, Department of Endodontics, National Key Laboratory of Oral Disease Prevention and Control & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Yang Xuetao, Master candidate, Department of Endodontics, National Key Laboratory of Oral Disease Prevention and Control & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
Supported by:National Natural Science Foundation of China, No. U21A20368 (to YL); China Postdoctoral Science Foundation Project, No. BX20230240 (to SYM); Sichuan Province Science and Technology Plan, No. 2024NSFSC1581 (to SYM)
摘要:
文题释义:
氧化应激:一般是指机体遭受各种有害刺激时,体内过氧化氢和羟基自由基等活性氧产生过多,超出了机体的抗氧化能力范围,氧化系统和抗氧化系统失衡,倾向于氧化,从而对机体产生负面作用,是导致衰老和疾病的一个重要因素。
抗氧化纳米材料:能够中和活性氧、改善氧化应激且三维空间尺度中至少有一维处于纳米量级的有机或无机材料。
背景:氧化应激与多种口腔疾病的发病有关,抗氧化纳米材料具有增强的活性氧清除特性,可以改善氧化应激,在口腔疾病中具有广阔的应用前景。
目的:总结近年来抗氧化纳米材料的研究进展以及在口腔疾病中的应用、不足和未来研究方向。
方法:以“reactive oxygen,antioxidant,nano,oxidative stress,oral”为关键词在PubMed和Web of Science数据库中检索文献,以“活性氧,抗氧化,纳米,氧化应激,口腔”为关键词在中国知网中检索文献,排除与研究主题关联性不强的文章,最终纳入103篇文献进行综述。
结果与结论:氧化应激是口腔多种疾病发病的机制之一,清除过量产生的活性氧并纠正氧化与抗氧化失调,是治疗口腔相关疾病的重要措施。抗氧化纳米材料因纳米结构特性具有高效的活性氧清除能力,能够改善机体氧化应激,促进相关疾病的恢复,在口腔相关疾病的研究应用中具有显著效果。抗氧化纳米材料合成原料昂贵、工艺复杂且在体内应用的长期安全性不明确,还需要进一步研究改进和验证。未来抗氧化纳米材料在口腔疾病中的研究应用需多学科交叉,同时结合大数据和人工智能等领域,在材料的设计、实验、应用和验证等方面进行优化,以实现安全、有效且舒适的个性化口腔疾病治疗。
https://orcid.org/0009-0006-7797-1173 (杨学涛)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
杨学涛, 朱梦菡, 张宸熙, 孙一民, 叶 玲. 抗氧化纳米材料在口腔中的应用和不足[J]. 中国组织工程研究, 2026, 30(8): 2044-2053.
Yang Xuetao, Zhu Menghan, Zhang Chenxi, Sun Yimin, Ye Ling. Applications and limitations of antioxidant nanomaterials in oral cavity[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2044-2053.
2.1.1 口腔疾病与氧化应激 氧化与抗氧化之间的微妙平衡对于维持口腔健康至关重要,氧化应激是口腔疾病的重要发病机制之一[9-11]。牙周病是受氧化应激影响最常见的口腔疾病之一[12]。在牙周病中,细菌感染会引起炎症,导致免疫细胞(如中性粒细胞和巨噬细胞)增多并产生较多活性氧[13-14],虽然活性氧水平增高对消除细菌具有重要作用[15],但是过高活性氧导致的氧化应激也造成了牙周支持组织的损伤和丧失[16]。临床研究发现,在牙周炎患者丙二醛和8-羟基脱氧鸟苷等氧化应激标志物水平增高[17]。龋齿也同样受到氧化应激的影响,变形链球菌等致龋细菌的代谢会促进活性氧的产生,过度增加的活性氧会损伤牙釉质和牙本质的有机质,同时诱导具有预防龋齿的唾液蛋白变性,加速龋病的进展[18-19]。研究证实,在口腔鳞状细胞癌中氧化应激标志物丙二醛和8-羟基脱氧鸟苷升高,并且升高水平和疾病预后密切相关[20]。 2.1.2 活性氧和氧化应激 活性氧是生物体内天然存在的含氧活性分子,主要由线粒体电子传递链和细胞质膜上的NADPH氧化酶复合体产生,部分由电离辐射等外界刺激产生,通常以氧自由基的形式存在[21-22]。常见的活性氧类型包括超氧阴离子、过氧化氢、单线态氧和羟基自由基等[23]。在生理状态下,活性氧在调节细胞的生长、分化、凋亡和基因表达等生理功能中发挥着重要作用[24]。
活性氧在机体内的生理水平主要依靠超氧化物歧化酶、过氧化氢酶和谷胱甘肽过氧化物酶等生物抗氧化酶以及维生素、类胡萝卜素和谷胱甘肽等抗氧化物调节[25-26]。然而,当机体受到病原体、损伤和炎症等刺激时,活性氧水平可能超过机体的调节能力,导致氧化与抗氧化平衡失调,从而引发氧化应激(图3)。氧化应激通过引起DNA碱基损伤和蛋白质结构与功能变性等方式诱导细胞损伤和凋亡,是多种疾病的共同发病机制[27]。
2.1.3 口腔疾病的传统治疗与抗氧化治疗 在口腔疾病治疗中,抗氧化主要是辅助传统治疗,以达到更好的治疗效果。传统的口腔疾病治疗主要是通过机械清创或者手术等方式去除导致疾病的直接因素,例如去除龋齿的龋坏组织、去除牙结石并消除细菌治疗牙周炎、手术切除癌变组织等,但是这些传统措施主要针对疾病的直接原因,并未及时缓解组织的损伤。相较于单纯的传统治疗,抗氧化辅助传统治疗可以从细胞、机制层面起作用,缓解氧化应激引起的损伤,加速疾病愈合的进展,因此,将抗氧化治疗与传统治疗相结合对于治愈氧化应激导致的口腔疾病至关重要。
2.1.4 针对口腔疾病氧化应激的措施 针对口腔疾病的氧化应激,目前临床上主要使用抗氧化剂消除过度增加的活性氧,常用的有辅酶Q10、维生素C、姜黄素和白藜芦醇等,然而,此类抗氧化剂在应用中又存在诸多问题[28]。随着纳米技术的不断发展,尤其是在抗氧化性能方向取得重大进展,具有抗氧化功能的纳米材料逐渐进入到生物医学研究视野[29],这些纳米材料具有特定的元素和结构,有望解决现有抗氧化剂的不足,维持更稳定的氧化与抗氧化平衡,进而恢复组织健康(表1)。
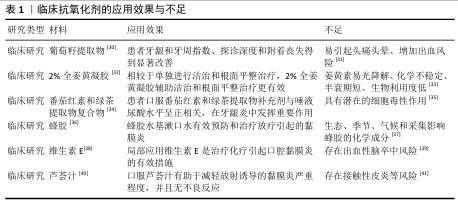
2.2 抗氧化纳米材料研究进展
2.2.1 纳米材料 纳米材料是尺寸介于原子、分子和宏观体系之间的纳米粒子组成的材料,其三维空间尺度至少有一维处于纳米量级(1-100 nm)。纳米材料具有更大的比表面积,因此,在吸附性能和催化性能方面具有更高的活性,同时纳米量级的尺寸可以赋予材料在光、热、声、电和磁等方面新的特性[42]。
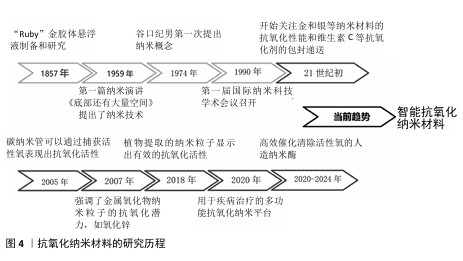
2.2.2 抗氧化纳米材料的发展 抗氧化纳米材料的发展经历了漫长的演变(图4)。19世纪中期,胶体化学开始建立和发展促使科学家们开始对直径100 nm以下的粒子体系进行研究。到了20世纪60年代,科学家们对分离的纳米粒子进行了研究。1974年,日本的NORIO TANIGUCHI教授首次使用“纳米技术”一词来描述精细的机械加工。1990年,美国召开了第一届国际纳米科技学术会议,正式宣布纳米材料科学是材料科学的一个新分支。
随着对疾病发病机制认识的逐步深入,对抗氧化的要求也越来越高,为了更有效治疗氧化应激相关疾病,纳米技术与抗氧化剂的融合成为了一个新的研究方向,并不断发展创新。在21世纪初,研究人员首次研究了脂质体和聚合物纳米粒子等用于维生素C、维生素E和辅酶Q10等抗氧化剂的包封递送。金、银和铈等金属纳米材料的抗氧化能力也引起了广泛关注,并在生物体中进行了实验应用。为了实现更全面的疾病治疗,众多研究致力于构建多功能纳米复合材料,在高效抗氧化的同时发挥促进骨、血管和神经的再生功能。ZHAN等[43]制备了一种负载有氮杂双二甲基磷酸酯封端的磷树枝状聚合物,并且使用具有活性氧响应性的纤维蛋白涂覆聚合物纳米粒子,在炎症氧化微环境中发挥抗氧化、抗炎和促进骨修复的功能。RASOOL 等[44]开发了一种硫醇化的生物活性介孔二氧化硅纳米粒子,能够中和细胞中的活性氧来保护细胞,促进成骨和抑制破骨细胞的生成,在骨质疏松中具有显著治疗效果。近几年研究人员通过分析并模拟过氧化氢酶和超氧化物歧化酶等天然酶的金属配位结构,合成了催化活性氧清除的人造纳米酶,这类酶具有更高效的活性氧清除特性,并且催化活性可再生,在体内具有稳定持久的活性氧清除特性。SUN等[45]通过模拟天然过氧化氢酶的铁-氮活性中心,将具有低电负性、更多d电子以及足够空轨道的钌引入金属配位结构,开发了一种比天然抗氧化剂和先前报道的活性氧清除催化剂更有效的人造仿酶材料,对干细胞保护具有重要意义。ZHANG等[46]模拟过氧化氢酶中的活性位点,报道了一种配备铂簇的抗氧化酶样生物催化剂,具有高效的催化清除活性氧能力,在牙周炎治疗中具有显著的效果。最近,更符合生物学过程的智能抗氧化纳米材料陆续出现。XU等[47]用透明质酸和聚多巴胺修饰具有催化性能的铂纳米粒子,同时制作了一个由水核和脂质壳组成的脂质体纳米反应器,通过水核包裹水溶性的尿激酶和铂纳米粒子级联双酶,脂质壳包含亲脂性的白藜芦醇,发挥清除有害的尿酸和过氧化物酶功能,改善缺氧的炎症免疫微环境,最后通过巨噬细胞衍生的外泌体和巨噬细胞膜伪装、靶向和溶酶体逃逸将药物输送到炎症部位发挥功能。
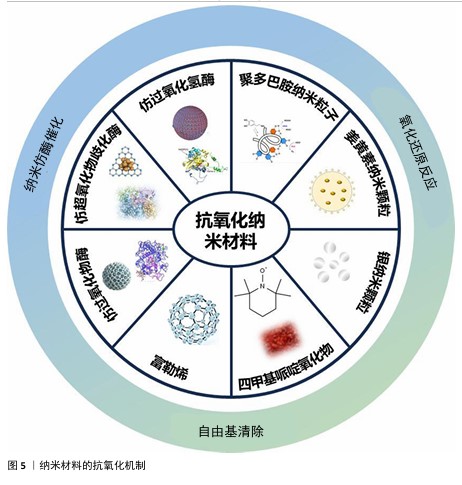
2.3 纳米材料的抗氧化机制 纳米材料的抗氧化机制见图5。
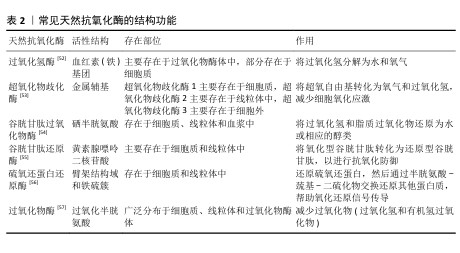
2.3.1 仿酶催化清除 机体自身主要有以下几种类型的氧化还原酶,包括过氧化物酶、过氧化氢酶和超氧化物歧化酶等,通过分析此类酶的化学结构,基于化学合成策略,衍生出了系列仿酶材料,这些纳米仿酶具有与天然酶类似的金属化学配位结构。受益于较大的比表面积,极小尺寸的纳米酶可以发挥高效、广谱的催化剂效果[48]。其中,类超氧化物歧化酶可以催化超氧阴离子歧化为氧气和过氧化氢,类过氧化氢酶可以将过氧化氢转换为氧气和水,类过氧化物酶能够还原过氧化物生成水。除此之外,部分纳米仿酶还可以通过电子供体的形式催化羟基自由基等自由基还原为水,从而减轻氧化应激[49-51]。常见天然抗氧化酶的结构功能,见表2。
2.3.2 氧化还原反应清除 自然界存在许多能够直接与活性氧反应来降低活性氧水平的物质。以姜黄素为基础的纳米粒子,能够通过自氧化消耗自由基,同时生成双环乙酰丙酮[58-59]。聚多巴胺纳米粒子邻位的醌结构可以发生H原子转移机制,与自由基直接进行反应[60]。银纳米粒子可以和超氧阴离子及羟基自由基等活性氧发生氧化还原反应,自身形成氧化银[61]。
2.3.3 自由基捕获 自由基捕获剂一般都是优良的电子受体,主要利用自身的单电子捕获氧自由基等自由基中不成对的电子,发生氧化还原反应,从而发挥抗氧化功能。2,2,6,6-四甲基哌啶氧化物是一种哌啶类氮氧自由基,氮氧化物/氧代铵氧化还原对通过可逆的单电子氧化还原反应促进催化过程,羟胺作为氢原子供体,提供抗氧化功能[62]。富勒烯(C60)是一种“自由基海绵”,具有良好电子亲和力的共轭双键,可以接受自由基不成对的电子从而猝灭各种自由基,改善氧化应激[63]。
2.4 抗氧化纳米材料在口腔中的应用与不足
2.4.1 抗氧化纳米材料在口腔中的应用
龋齿:是口腔中发病率很高的疾病,可能与唾液氧化生物化学的变化及脂质过氧化增加有关。HENDI 等[64]通过临床试验发现,龋齿活跃的志愿者唾液中过氧化物酶、尿酸、过氧化氢酶和谷胱甘肽过氧化物酶水平较高,超氧化物歧化酶水平较低。研究表明,硒纳米颗粒具有抗氧化和抗菌效果,可能具有抗染色和预防龋齿的效果[65]。
牙髓炎:龋病、磨损和创伤等因素都有可能导致不可复性牙髓炎症,目前临床针对不可复性牙髓炎的治疗方式主要是将牙髓杀死并去除,但失去牙髓营养的牙齿会变得更脆弱,同时失去感知功能[66]。人源牙髓干细胞具有高度繁殖、自我更新及多向分化潜能,目前已有相关研究将其移植在根管中再生牙髓,但是由于根管特殊的解剖结构,移植的牙髓干细胞容易发生急性缺氧[67]。香港大学利用低氧环境激发用作移植牙髓干细胞的保护性反应——氧化应激反应,增强移植干细胞的根管适应能力[68]。虽然这类方法能在一定程度上提高牙髓干细胞的适应能力,但是低氧环境条件并不完全与根管环境相同,并且无法解决移植后炎症可能再次发作的问题。ZHAO等[69]将具有抗氧化的二氧化铈纳米粒子和促进牙本质再生的牙本质基质蛋白1负载到可注射的Fmoc-三苯丙氨酸水凝胶中,将该水凝胶注入根管中发挥抗氧化、抗炎和促进牙本质生成的作用。
牙周炎:是由菌斑和牙结石等局部刺激因素引起的牙周支持组织破坏性疾病。病原微生物是破坏牙周组织的始动因子,机体免疫炎症反应也起到了重要作用,炎症状态下的人牙周组织会产生过量的活性氧物质,出现氧化应激。当前牙周炎的治疗以去除感染因素为主,通过龈上洁治、龈下刮治等物理疗法和局部使用派力奥等抗菌剂辅助治疗,对于较为严重的牙周炎,临床上还采用牙龈切除术、翻瓣术等手段进一步治疗,以尽可能去除感染因素,形成不利于牙菌斑堆积的解剖结构。然而,牙周炎造成的牙槽骨缺损往往难以恢复,导致患牙抵抗力差。因此,患者需要更加重视牙周健康的维护,以避免复发和牙齿进一步松动脱落[70]。临床研究表明,抗氧化疗法作为牙周病的单一疗法或者辅助治疗可以减少牙周炎症[71]。HUANG等[72]用阳离子聚酰胺胺树枝状大分子涂覆含硒的羟基磷灰石纳米颗粒,开创了一种清除与牙周炎高度相关的循环游离DNA的纳米材料,不仅可以抑制循环游离DNA的体外促炎作用,还可以缓解牙周炎症性骨丢失。XU等[73]提出了一种模拟超氧化物歧化酶和过氧化氢酶的铜基纳米酶,通过与甘油单硬酸酯和2,6-二叔丁基-4-甲基苯酚组装的水凝胶结合,实现牙周炎部位纳米酶的按需释放,便捷有效地缓解牙周炎症,促进组织再生。
口腔癌:口腔受到各种致癌因素刺激后,口腔黏膜中的活性氧会过度产生,损伤DNA和蛋白质等,导致突变和癌变。除此以外,活性氧增加还会降低T细胞和自然杀伤细胞的功能,促进巨噬细胞的募集、M2极化和肿瘤的发展[74]。其中,口腔鳞状细胞癌肿瘤微环境就以氧化应激为主要特征,不仅促进癌细胞存活增殖,而且不利于化疗和放疗。因此,抗氧化策略可能是治疗口腔癌的一种潜在方式。FAZLI等[75]将姜黄素和囊泡结合制备的纳米囊泡注射入动物体内发挥抗氧化动能,结果显示可有效防止严重形式的发育不良发展并抑制癌细胞的生长。然而,目前也有大量研究通过纳米材料产生大量活性氧抑制或杀死肿瘤细胞,用于口腔癌症或其他癌症的治疗。LUCKY等[76]开发了以近红外光激发的上转化纳米颗粒为基础的光动力纳米材料,可以特异性靶向过表达口腔癌细胞的上皮因子受体,在光照下产生活性氧,实现口腔癌细胞的破坏。ZHONG等[77]尝试在各种温度下煅烧控制钌纳米晶格的晶格间距以调节钌基纳米酶的电子状态,最大限度地提高纳米酶催化活性氧产生的能力,实现最佳的肿瘤治疗。活性氧在肿瘤中具有两面性,既可以表现为促进肿瘤发生,也可以抑制肿瘤。GLORIEUX等[78]通过总结众多研究结果认为,不同研究者得出的结论不同甚至相互矛盾可能是由于不同研究模型和方法造成的。活性氧的时空调控及在肿瘤发展不同阶段的动态效应,可能在一定程度上解释抗氧化剂在肿瘤中的作用。因此,如何利用活性氧治疗口腔癌症,需要从口腔癌症的具体类型、发展状态、分期分级及患者自身实际状况等方面综合考虑,制定出合理的活性氧治疗方案。
扁平苔藓:口腔扁平苔藓是口腔中常见的一种黏膜病变,主要表现为小丘疹样病损组成的线状、网状、树枝状或者斑块状病变,在中年女性中发病较高。相关研究表明,口腔扁平苔藓的发病机制与氧化应激相关[79],活性氧会诱导细胞凋亡,而角质形成细胞的凋亡是口腔扁平苔藓的标志[80]。系统评价结果表明,抗氧化治疗可以减轻口腔扁平苔藓患者的疼痛和临床评分,提高缓解率[81]。
ALIPOUR等[82]设计并制备了一种含有霉酚酸酯、氧化锌纳米粒子和芦荟的纳米纤维垫,患有双侧红斑/糜烂性口腔扁平苔藓的患者使用后与临床用的软膏疗效相同,并且症状得到明显改善。
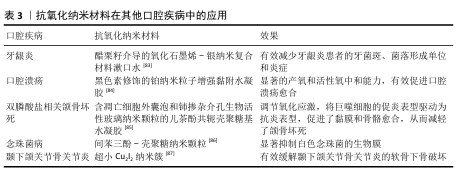
除了龋齿、牙髓炎、牙周炎和口腔癌等口腔疾病,抗氧化纳米材料在其他口腔疾病中也有广泛应用(表3)。
2.4.2 抗氧化纳米材料口腔应用不足
潜在毒性:大多数具有活性氧清除效果的抗氧化纳米材料都含有过渡金属元素,在材料合成过程中的金属和杂质残留均容易引起生物毒性。纳米尺寸的材料容易进入全身循环系统,在肝脾等脏器积聚,同时也可能进入细胞而造成细胞凋亡。不同抗氧化纳米材料还具有丰富多样的形状,例如纳米球、纳米棒、纳米片等,不同形状的纳米材料可能位于细胞外,也可能锚定在细胞上或进入细胞,对细胞活力造成影响[88-89]。在过去的10年中,人们一直在探索纳米材料对生物系统的影响,纳米材料对细胞毒性的作用是动态的[90]。因此,系统评估抗氧化纳米材料的生物安全性至关重要,需要从合成原料、合成工艺以及纯化方面不断改进,从细胞、组织、个体和群体等方面长期综合评估抗氧化纳米材料的安全性[91]。
合成复杂:抗氧化纳米材料的合成需要昂贵的原材料、复杂的程序、精密的仪器设备和控制技术,并且纳米材料的形态、结构、电荷及成分都会影响材料的特性,最终影响细胞行为。目前大多数抗氧化纳米材料的合成都是通过不断试错筛选制备的,找到一种既具良好活性氧清除性能又能满足口腔组织相应部位效果和安全性的纳米材料很难[92]。
口腔保留稳定性:口腔是一个湿润的环境,不断有唾液分泌,并且龈沟内充满龈沟液,在说话咀嚼和吞咽时伴随骨、关节、肌肉和黏膜的运动,单纯抗氧化纳米材料应用于口腔时难以在疾病部位保持稳定,很容易流失或进入循环,因此,不得不增加口腔给药的频率,这可能带来潜在的生物毒性[93]。虽然目前也有许多研究将纳米抗氧化剂放入凝胶等聚合物材料中保持其在病变部位的稳定性,但是这种组合可能会对纳米抗氧化剂自身的性能产生影响。
2.5 口腔抗氧化纳米材料研究前沿
2.5.1 智能抗氧化纳米材料 生理水平的活性氧对于维持机体的正常功能是必须的,常常作为信号分子参与调控生物学过程,包括激活信号通路、调控细胞生长和影响生物体器官等。但是目前的抗氧化纳米材料只能单纯发挥抗氧化作用,并不能根据生理所需调控活性氧水平。当氧化应激解除以后,残留的抗氧化纳米材料可能继续清除生理水平的活性氧,影响正常的生理功能。除此以外,目前已经研究出了能够反复清除活性氧的纳米酶材料,这类材料随着循环进入到正常组织后仍然可以保持活性氧清除功能,对正常组织可能造成危害。
因此,未来可以尝试开发在具体疾病微环境中经过特定刺激激活抗氧化特性的智能纳米材料,避免对正常生理状态的干扰。除此以外,也可以将人工智能结合到纳米技术和生物医学领域,通过程序设计和计算机模拟、高通量筛选探索出更加科学的抗氧化纳米材料,同时借助人工智能收集抗氧化纳米材料应用在生物体中的各项实时生理参数,以便更准确的分析[94-95]。
2.5.2 安全容易代谢纳米材料 目前绝大多数抗氧化纳米材料都包含金属元素,例如银和锰等,同时纳米级尺寸使得材料更容易被细胞吞噬和进入全身循环系统,在肝脏和脾脏等部位聚集,对健康产生潜在的危害[96]。目前也有许多提高抗氧化纳米材料生物相容性的方法,例如,LI等[97]将多个乙二胺基团键合到富勒烯上获得水溶性的纳米粒子,不仅具有优异的活性氧清除能力,同时也具有良好的生物相容性;也有研究利用红细胞和中性粒细胞等细胞的胞膜对抗氧化纳米材料进行涂层,以改善其性能和生物安全性[98]。
但是从抗氧化纳米材料本身设计出发,构建自身更安全高效的抗氧化纳米材料,特别是容易被机体安全代谢并排泄的纳米材料,是未来需要不断努力的方向。
2.5.3 个性化抗氧化纳米材料 个性化医疗在近10年来取得了很大发展,尤其是在靶向药物等方面的突破,个性化医疗根据患者可监测的遗传信息和环境信息量身定做治疗计划,实现治疗效果最大化和不良反应最小化[99]。
对于不同患者、不同疾病状态以及不同的疾病阶段,氧化应激情况可能都不同,对药物的代谢能力也不同。为了尽可能发挥纳米材料的抗氧化效果以及减轻生物毒性,抗氧化纳米材料需要朝着更加精准的方向发展,具体可以从以下几个方面努力:①量身定制:根据不同的疾病类型及其氧化应激状态,从材料组分、给药方式和剂量等方面量身定制抗氧化纳米材料;②精准靶向:通过材料设计实现抗氧化纳米材料靶向特定部位或者细胞;③多学科交叉:促进医学、材料学、生物学和化学等领域的专家团队合作,创新改进抗氧化纳米材料治疗方式。
| [1] ROWIŃSKA I, SZYPERSKA-ŚLASKA A, ZARICZNY P, et al. The influence of diet on oxidative stress and inflammation induced by bacterial biofilms in the human oral cavity. Materials (Basel). 2021;14(6):1444. [2] SARDARO N, DELLA VELLA F, INCALZA MA, et al. Oxidative stress and oral mucosal diseases: an overview. In Vivo. 2019;33(2):289-296. [3] BIRBEN E, SAHINER UM, SACKESEN C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9-19. [4] LANDETE JM. Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr. 2013;53(7):706-721. [5] LIU X, XU H, PENG H, et al. Advances in antioxidant nanozymes for biomedical applications. Coordin Chem Rev. 2024;502: 215610. [6] ABEDI N, SAJADI-JAVAN Z S, KOUHI M, et al. Antioxidant materials in oral and maxillofacial tissue regeneration: a narrative review of the literature. Antioxidants (Basel). 2023; 12(3):594. [7] SUI L, WANG J, XIAO Z, et al. ROS-scavenging nanomaterials to treat periodontitis. Front Chem. 2020;8:595530. [8] KUMAR H, BHARDWAJ K, NEPOVIMOVA E, et al. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials (Basel). 2020;10(7):1334. [9] KESARWALA AH, KRISHNA MC, MITCHELL JB. Oxidative stress in oral diseases. Oral Dis. 2016;22(1):9-18. [10] MEYLE J, CHAPPLE IJP. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. 2015;69(1):7-17. [11] 王雨,刘佳.氧化应激与口腔炎性疾病的相关性综述[J].现代口腔医学杂志, 2024,38(1):66-69. [12] 郑燕丹,黄翔.活性氧在牙周炎中病理作用的研究进展[J].安徽医药,2019,23(7): 1295-1298. [13] CEKICI A, KANTARCI A, HASTURK H, et al. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64(1):57-80. [14] CANAKCI C, CICEK Y, CANAKCI VJB. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry (Mosc). 2005;70(6):619-628. [15] NATHAN C, CUNNINGHAM-BUSSEL A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349-361. [16] SCZEPANIK FSC, GROSSI ML, CASATI M, et al. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 2000. 2020;84(1):45-68. [17] FENTOĞLU Ö, KIRZIOĞLU F Y, BULUT MT, et al. Evaluation of lipid peroxidation and oxidative DNA damage in patients with periodontitis and hyperlipidemia. J Periodontol. 2015;86(5):682-688. [18] DE SOUSA NÉ YG, LIMA WF, MENDES PFS, et al. Dental caries and salivary oxidative stress: global scientific research landscape. Antioxidants (Basel). 2023;12(2):330. [19] SOUTHWARD K. The systemic theory of dental caries. Gen Dent. 2011;59(5): 367-373;quiz 374-5. [20] AGHA‐HOSSEINI F, MIRZAII‐DIZGAH I, FARMANBAR N, et al. Oxidative stress status and DNA damage in saliva of human subjects with oral lichen planus and oral squamous cell carcinoma. J Oral Pathol Med. 2012;41(10):736-740. [21] MATOUGH FA, BUDIN SB, HAMID ZA, et al. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12(1):5-18. [22] ŻUKOWSKI P, MACIEJCZYK M, WASZKIEL D. Sources of free radicals and oxidative stress in the oral cavity. Arch Oral Biol. 2018;92:8-17. [23] SCHIEBER M, CHANDEL NAVDEEP S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453-R462. [24] SIES H, BELOUSOV VV, CHANDEL NS, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23(7):499-515. [25] JOMOVA K, RAPTOVA R, ALOMAR SY, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch Toxicol. 2023;97(10):2499-2574. [26] MATEEN S, MOIN S, KHAN AQ, et al. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. 2016;11(4):e0152925. [27] HAJAM YA, RANI R, GANIE SY, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. 2022;11(3):552. [28] RATNAM DV, ANKOLA DD, BHARDWAJ V, et al. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 2006; 113(3):189-207. [29] KUMAR H, BHARDWAJ K, NEPOVIMOVA E, et al. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials (Basel). 2020;10(7):1334. [30] DAS M, DAS AC, PANDA S, et al. Clinical efficacy of grape seed extract as an adjuvant to scaling and root planing in treatment of periodontal pockets. J Biol Regul Homeost Agents. 2021;35:89-96. [31] BERRY AC, NAKSHABENDI R, ABIDALI H, et al. Adverse effects of grape seed extract supplement: a clinical case and long-term follow-up. J Diet Suppl. 2016;13(2):232-235. [32] BEHAL R, MALI AM, GILDA SS, et al. Evaluation of local drug-delivery system containing 2% whole turmeric gel used as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study. J Indian Soc Periodontol. 2011;15(1):35-38. [33] KARTHIKEYAN A, SENTHIL N, MIN T. Nanocurcumin: a promising candidate for therapeutic applications. Front Pharmacol. 2020;11:487. [34] WASTI J, WASTI A, SINGH R. Efficacy of antioxidants therapy on progression of periodontal disease - A randomized control trial. Indian J Dent Res. 2021;32(2):187-191. [35] QU M, ZHOU Z, CHEN C, et al. Lycopene protects against trimethyltin-induced neurotoxicity in primary cultured rat hippocampal neurons by inhibiting the mitochondrial apoptotic pathway. Neurochem Int. 2011;59(8):1095-1103. [36] JAVADZADEH BOLOURI A, PAKFETRAT A, TONKABONI A, et al. Preventing and therapeutic effect of propolis in radiotherapy induced mucositis of head and neck cancers: a triple-blind, randomized, placebo-controlled trial. Iran J Cancer Prev. 2015;8(5):e4019. [37] SFORCIN JM, BANKOVA V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133(2): 253-260. [38] EL-HOUSSEINY AA, SALEH SM, EL-MASRY AA, et al. The effectiveness of vitamin “E” in the treatment of oral mucositis in children receiving chemotherapy. J Clin Pediatr Dent. 2007;31(3):167-170. [39] MAGGIO E, BOCCHINI VP, CARNEVALE R, et al. Vitamin E supplementation (alone or with other antioxidants) and stroke: a meta-analysis. Nutr Rev. 2024;82(8):1069-1078. [40] AHMADI A. Potential prevention: Aloe vera mouthwash may reduce radiation-induced oral mucositis in head and neck cancer patients. Chin J Integr Med. 2012;18(8):635-640. [41] FERREIRA M, TEIXEIRA M, SILVA E, et al. Allergic contact dermatitis to Aloe vera. Contact Dermatitis. 2007;57(4):278-279. [42] SAHLE FF, GULFAM M, LOWE TL. Design strategies for physical-stimuli-responsive programmable nanotherapeutics. Drug Discov Today. 2018;23(5):992-1006. [43] ZHAN M, SUN H, WANG Z, et al. Nanoparticle-mediated multiple modulation of bone microenvironment To tackle osteoarthritis. ACS Nano. 2024; 18(15):10625-10641. [44] RASOOL N, NEGI D, SINGH Y. Thiol-functionalized, antioxidant, and osteogenic mesoporous silica nanoparticles for osteoporosis. ACS Biomater Sci Eng. 2023; 9(6):3535-3545. [45] SUN Y, MU S, XING Z, et al. Catalase-mimetic artificial biocatalysts with Ru catalytic centers for ROS elimination and stem-cell protection. Adv Mater. 2022; 34(46):e2206208. [46] ZHANG C, YAN R, BAI M, et al. Pt-clusters-equipped antioxidase-like biocatalysts as efficient ROS scavengers for treating periodontitis. Small. 2024;20(17):e2306966. [47] XU J, WU M, YANG J, et al. Multimodal smart systems reprogramme macrophages and remove urate to treat gouty arthritis. Nat Nanotechnol. 2024;19(10):1544-1557. [48] YANG W, YANG X, ZHU L, et al. Nanozymes: Activity origin, catalytic mechanism, and biological application. Coordin Chem Rev. 2021;448:214170. [49] ZHAO H, ZHANG R, YAN X, et al. Superoxide dismutase nanozymes: an emerging star for anti-oxidation. J Mater Chem B. 2021; 9(35):6939-6957. [50] XU D, WU L, YAO H, et al. Catalase-like nanozymes: Classification, catalytic mechanisms, and their applications. Small. 2022;18(37):2203400. [51] ATTAR F, SHAHPAR MG, RASTI B, et al. Nanozymes with intrinsic peroxidase-like activities. J Mol Liq. 2019;278:130-144. [52] DÍAZ A, LOEWEN PC, FITA I, et al. Thirty years of heme catalases structural biology. Arch Biochem Biophys. 2012;525(2): 102-110. [53] FUKAI T, USHIO-FUKAI M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583-1606. [54] WEAVER K, SKOUTA R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines. 2022;10(4): 891. [55] COUTO N, WOOD J, BARBER J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med. 2016;95:27-42. [56] LU J, HOLMGREN A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75-87. [57] SHEN C, WANG Y. Recent progress on peroxidase modification and application. Appl Biochem Biotechnol. 2024;196(9): 5740-5764. [58] DOGGUI S, SAHNI JK, ARSENEAULT M, et al. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J Alzheimers Dis. 2012;30(2):377-392. [59] GRIESSER M, PISTIS V, SUZUKI T, et al. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J Biol Chem. 2011;286(2):1114-1124. [60] GUO Y, BASCHIERI A, MOLLICA F, et al. Hydrogen Atom Transfer from HOO. to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew Chem Int Ed Engl. 2021;60(28):15220-15224. [61] BEDLOVIČOVÁ Z, STRAPÁČ I, BALÁŽ M, et al. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules. 2020;25(14):3191. [62] SOULE BP, HYODO F, MATSUMOTO KI, et al. The chemistry and biology of nitroxide compounds. Free Radical Biology and Medicine. Free Radic Biol Med. 2007; 42(11):1632-1650. [63] LIN AMY, CHYI BY, WANG SD, et al. Carboxyfullerene prevents iron-induced oxidative stress in rat brain. J Neurochem. 1999;72(4):1634-1640. [64] HENDI SS, GOODARZI MT, MOGHIMBEIGI A, et al. Evaluation of the status of salivary antioxidants in dental caries. Infect Disord Drug Targets. 2020;20(6):816-821. [65] ALMUQRIN A, KAUR IP, WALSH LJ, et al. Amelioration strategies for silver diamine fluoride: moving from black to white. Antibiotics (Basel). 2023;12(2):298. [66] YAN W, MONTOYA C, ØILO M, et al. Contribution of root canal treatment to the fracture resistance of dentin. J Endod. 2019;45(2):189-193. [67] DISSANAYAKA WL, HARGREAVES KM, JIN L, et al. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A. 2015;21(3-4):550-563. [68] HAN Y, KOOHI-MOGHADAM M, CHEN Q, et al. HIF-1α stabilization boosts pulp regeneration by modulating cell metabolism. J Dent Res. 2022;101(10): 1214-1226. [69] ZHAO Y, SONG L, LI M, et al. Injectable CNPs/DMP1-loaded self-assembly hydrogel regulating inflammation of dental pulp stem cells for dentin regeneration. Materials Today Bio. 2023;24:100907. [70] ONUORA S. NETs implicated in periodontitis-associated bone loss. Nat Rev Rheumatol. 2023;19(8):463. [71] MATHUR A, MATHUR L, MANOHAR B, et al. Antioxidant therapy as monotherapy or as an adjunct to treatment of periodontal diseases. J Indian Soc Periodontol. 2013; 17(1):21-24. [72] HUANG H, PAN W, WANG Y, et al. Nanoparticulate cell-free DNA scavenger for treating inflammatory bone loss in periodontitis. Nat Commun. 2022;13(1): 5925. [73] XU Y, LUO Y, WENG Z, et al. Microenvironment-responsive metal-Phenolic nanozyme release platform with antibacterial, ROS scavenging, and osteogenesis for periodontitis. ACS Nano. 2023;17(19):18732-18746. [74] HUANG R, CHEN H, LIANG J, et al. Dual role of reactive oxygen species and their application in cancer therapy. J Cancer. 2021;12(18):5543-5561. [75] FAZLI B, IRANI S, BARDANIA H, et al. Prophylactic effect of topical (slow-release) and systemic curcumin nano-niosome antioxidant on oral cancer in rat. BMC Complement Med. 2022;22(1):109. [76] LUCKY SS, IDRIS NM, HUANG K, et al. In vivo biocompatibility, biodistribution and therapeutic efficiency of titania coated upconversion nanoparticles for photodynamic therapy of solid oral cancers. Theranostics. 2016;6(11):1844-1865. [77] ZHONG S, ZHANG Z, ZHAO Q, et al. Lattice expansion in ruthenium nanozymes improves catalytic activity and electro-responsiveness for boosting cancer therapy. Nat Commun. 2024;15(1):8097. [78] GLORIEUX C, LIU S, TRACHOOTHAM D, et al. Targeting ROS in cancer: rationale and strategies. Nat Rev Drug Discov. 2024; 23(8):583-606. [79] UPADHYAY RB, CARNELIO S, SHENOY RP, et al. Oxidative stress and antioxidant defense in oral lichen planus and oral lichenoid reaction. Scand J Clin Lab Invest. 2010;70(4):225-228. [80] SANKARI SL, BABU NA, RAJESH E, et al. Apoptosis in immune-mediated diseases. J Pharm Bioallied Sci. 2015;7:S200-202. [81] BAO J, CHEN C, YAN J, et al. Antioxidant therapy for patients with oral lichen planus: A systematic review and meta-analysis. Front Pharmacol. 2022;13:1030893. [82] ALIPOUR M, HABIBIVAND E, SEKHAVATI S, et al. Evaluation of therapeutic effects of nanofibrous mat containing mycophenolate mofetil on oral lichen planus: In vitro and clinical trial study. Biomater Investig Dent. 2023;10(1):2283177. [83] SOUNDARAJAN S, RAJASEKAR AJD, PROBLEMS M. Antibacterial and anti-inflammatory effects of a novel herb-mediated nanocomposite mouthwash in plaque-induced gingivitis: a randomized controlled trial. Dent Med Probl. 2023;60(3): 445-451. [84] CAI E, QI X, SHI Y, et al. Immunomodulatory melanin@Pt nanoparticle-reinforced adhesive hydrogels for healing diabetic oral ulcers. Chem Eng J. 2024;488:150372. [85] LING Z, GUO S, XIE H, et al. Synergistic effects of cerium-containing bioactive glasses and apoptotic extracellular vesicles alleviate bisphosphonate-related osteonecrosis of jaw. Appl Mater Today. 2024;38:102177. [86] KHAN F, OH D, CHANDIKA P, et al. Inhibitory activities of phloroglucinol-chitosan nanoparticles on mono- and dual-species biofilms of Candida albicans and bacteria. Colloids Surf B Biointerfaces. 2022;211:112307. [87] WANG Y, DING X, ZHANG F, et al. Ultrasmall Cu2I2 nanoclusters trigger metabolic-epigenetic reprogramming and endogenous antioxidant systems for alleviating osteoarthritis. Chem Eng J. 2024;497:154568. [88] MEDICI S, PEANA M, PELUCELLI A, et al. An updated overview on metal nanoparticles toxicity. Semin Cancer Biol. 2021;76:17-26. [89] HE X, FU P, AKER WG, et al. Toxicity of engineered nanomaterials mediated by nano-bio-eco interactions. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2018;36(1):21-42. [90] EFTEKHARI A, DIZAJ SM, CHODARI L, et al. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed Pharmacother. 2018;103:1018-1027. [91] YAN L, ZHAO F, WANG J, et al. A safe-by-design strategy towards safer nanomaterials in nanomedicines. Adv Mater. 2019;31(45): 1805391. [92] YANG L, DONG S, GAI S, et al. Deep insight of design, mechanism, and cancer theranostic strategy of nanozymes. Nanomicro Lett. 2023;16(1):28. [93] CHEN Z, CHU Z, JIANG Y, et al. Recent advances on nanomaterials for antibacterial treatment of oral diseases. Materials Today Bio. 2023;20:100635. [94] WANG Z, WU J, ZHENG JJ, et al. Accelerated discovery of superoxide-dismutase nanozymes via high-throughput computational screening. Nat Commun. 2021;12(1):6866. [95] KLEANDROVA VV, LUAN F, GONZÁLEZ-DÍAZ H, et al. Computational tool for risk assessment of nanomaterials: novel QSTR-perturbation model for simultaneous prediction of ecotoxicity and cytotoxicity of uncoated and coated nanoparticles under multiple experimental conditions. Environ Sci Technol. 2014;48(24):14686-14694. [96] SANCHEZ VC, PIETRUSKA JR, MISELIS NR, et al. Biopersistence and potential adverse health impacts of fibrous nanomaterials: what have we learned from asbestos? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(5):511-529. [97] LI J, GUAN M, WANG T, et al. Gd@C(82)-(ethylenediamine)(8) nanoparticle: A new high-efficiency water-soluble ROS scavenger. ACS Appl Mater Interfaces. 2016;8(39): 25770-25776. [98] LIU J, MA L, ZHANG G, et al. Recent progress of surface modified nanomaterials for scavenging reactive oxygen species in organism. Bioconjug Chem. 2021;32(11): 2269-2289. [99] JOHNSON KB, WEI WQ, WEERARATNE D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. [100] ABEDI N, SAJADI-JAVAN ZS, KOUHI M, et al. Antioxidant materials in oral and maxillofacial tissue regeneration: A narrative review of the literature. Antioxidants (Basel). 2023;12(3):594. [101] BENZIAN H, WATT R, MAKINO Y, et al. WHO calls to end the global crisis of oral health. Lancet. 2022;400(10367): 1909-1910. [102] JAIN N, DUTT U, RADENKOV I, et al. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2024;30(2):73-79. [103] BELIBASAKIS GN, BOSTANCI N, MARSH PD, et al. Applications of the oral microbiome in personalized dentistry. Arch Oral Biol. 2019;104:7-12. |
| [1] | 陈豪杰, 王 黛, 沈 山. 种植体周围炎中的免疫炎症微环境机制[J]. 中国组织工程研究, 2026, 30(8): 2054-2062. |
| [2] | 杨琼琼, 刘 玮. 氧化锆与钛种植体的性能及临床效果对比[J]. 中国组织工程研究, 2026, 30(8): 2063-2071. |
| [3] | 刘大为, 崔颖颖, 王方辉, 王子轩, 陈宇涵, 李友瑞, 张荣和. 表没食子儿茶素没食子酸酯介导活性氧双向调控及在纳米材料中的应用[J]. 中国组织工程研究, 2026, 30(8): 2101-2112. |
| [4] | 刘宏杰, 牟秋菊, 申玉雪, 梁 飞, 祝丽丽. 金属有机框架/羧甲基壳聚糖-氧化海藻酸钠/富血小板血浆水凝胶促糖尿病感染创面愈合[J]. 中国组织工程研究, 2026, 30(8): 1929-1939. |
| [5] | 郑旭颖, 胡洪成, 许礼兵, 韩建民, 邸 萍. 不同载荷形式和内连接形状下两段式粘接固位氧化锆种植体的应力大小和分布[J]. 中国组织工程研究, 2026, 30(8): 1979-1987. |
| [6] | 董春阳, 周天恩, 莫孟学, 吕文权, 高 明, 朱瑞凯, 高志伟. 二甲双胍联合血水草敷料治疗深Ⅱ度烧伤创面的作用机制[J]. 中国组织工程研究, 2026, 30(8): 2001-2013. |
| [7] | 王菘芃, 刘玉三, 于焕英, 高晓丽, 徐英江, 张晓明, 刘 敏. 沸石基咪唑盐框架8纳米材料的活性氧双向调控:从肿瘤治疗、抗菌到细胞保护[J]. 中国组织工程研究, 2026, 30(8): 2033-2013. |
| [8] | 白相宇, 霍 峰, 郝 妍, 王泽成, 郭晓宇. 负载血小板衍生生长因子BB的壳聚糖/还原氧化石墨烯支架修复牙槽骨缺损[J]. 中国组织工程研究, 2026, 30(2): 329-337. |
| [9] | 董 超, 赵漠涵, 刘宇楠, 杨泽丽, 陈乐琴, 王兰芳. 磁性纳米药物载体对大鼠运动性肌肉损伤及炎性反应的影响[J]. 中国组织工程研究, 2026, 30(2): 345-353. |
| [10] | 王宇航, 张 涵, 张超晶, 寇绪容, 井桐桐, 林日梅, 刘鑫宇, 娄石磊, 阎 慧, 孙 聪. 姜黄素提取及姜黄素纳米粒的制备及优化[J]. 中国组织工程研究, 2026, 30(2): 362-374. |
| [11] | 李锐强, 尹 晨, 马 琰. 过氧化脲和过氧化氢两种漂白剂对牙本质拉曼光谱激光诱导荧光的影响[J]. 中国组织工程研究, 2026, 30(2): 296-302. |
| [12] | 赵春红, 何 俐. 三种机用镍钛器械以不同技术预备弯曲根管的效果比较[J]. 中国组织工程研究, 2026, 30(2): 303-309. |
| [13] | 程亚男, 于佳志, 刘印倡, 吴 杰, 于 彤, 王 璐, 李晓光. 不同厚度与边缘无托槽隐形矫治器推磨牙远移的三维有限元分析[J]. 中国组织工程研究, 2026, 30(2): 310-318. |
| [14] | 亢紫瑞, 武 洋, 宋海龙, 杨巧芸, 臧理想, 许东亮. 不同冠根比种植体在不同骨质下的有限元分析[J]. 中国组织工程研究, 2026, 30(2): 319-328. |
| [15] | 张其娅, 童伊翔, 杨世姣, 张宇梦, 邓 凌, 吴 玮, 解 瑶, 廖 健, 毛 岭. 梯度玻璃分级超透氧化锆的体外生物相容性[J]. 中国组织工程研究, 2026, 30(2): 443-450. |
目前,针对龋病、牙髓炎、牙周炎和口腔癌等口腔疾病的抗氧化纳米材料研究有很多,并且都证实了抗氧化纳米材料在缓解氧化应激改善疾病状态中的显著效果。然而,也有许多报道表明部分抗氧化纳米材料存在潜在的毒性等问题,尤其是具有独特性质及含有金属元素的纳米材料[5-8]。但是,随着纳米技术的不断发展,抗氧化纳米材料也在不断改进完善。
为了系统认识口腔疾病氧化应激及抗氧化纳米材料的应用、不足和未来,该文基于抗氧化纳米材料在口腔疾病治疗中的相关研究,探讨其在口腔治疗中面临的问题及研究现状,为抗氧化纳米材料的临床转化研究提供新思路。
1.1.1 检索人及检索时间 由第一作者于2024年9月进行检索。
1.1.2 文献检索时限 各数据库建库至2024年9月。
1.1.3 检索数据库 中国知网、PubMed和Web of Science数据库。
1.1.4 检索词 英文检索词“reactive oxygen,antioxidant,nano,oxidative stress,oral”,中文检索关键词“活性氧,抗氧化,纳米,氧化应激,口腔”。
1.1.5 检索文献类型 研究原著和综述。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以PubMed数据库检索策略为例,见图1。
1.1.8 检索文献量 共检索文献540篇,其中PubMed数据库187篇、Web of Science数据库264篇、中国知网89篇。
1.2 入选标准
纳入标准:①与抗氧化纳米材料在口腔中应用相关的研究;②具有原创性、逻辑严密、数据完整的研究;③发表在权威杂志或被多次引用的文章。
排除标准:①与研究内容无关、数据支持性不足的文献;②逻辑不完整、结论不客观的研究;③研究内容重复性文章。
1.3 文献质量评估及数据的提取 通过数据库共检索文献540篇,通过文章标题、摘要、关键词和背景介绍进行筛选,排除与主题关系不大、原创性不足以及数据说服力不够的文献,最终纳入103篇进行综述。文献检索流程见图2。
3.2 该综述区别于他人的特点 近年来关于纳米材料的综述多集中于全身疾病的治疗,尤其是肿瘤、炎症和心脑血管疾病的治疗研究。口腔的氧化应激研究重点集中在机制通路方面,对于抗氧化纳米材料在口腔领域的研究应用综述还比较欠缺。该文从口腔疾病与氧化应激的关系以及抗氧化纳米材料研究现状出发,总结了近十几年抗氧化纳米材料在口腔领域的研究应用,尤其是分析了目前抗氧化纳米材料所面临的问题,结合未来健康需要、现有研究基础和临床应用特点,提出了口腔抗氧化纳米材料的未来研究方向,希望为后续的抗氧化纳米材料研究提供一些思路,促进临床转化。
3.3 该综述的局限性 综述是对别人研究结果的总结和论述,非常依赖于实验结果的准确客观性,而研究的实验条件、建模标准和处理方式还不能做到大范围内的统一,这也是目前许多研究结论存在争议的地方,可能影响综述的结论。该文论述了抗氧化纳米材料在口腔中的应用效果和不足,没有对相关的生物学机制进行深入阐述,但是文章已经大致介绍了部分常见的抗氧化生物学机制,同时对抗氧化纳米材料的作用机制也进行了大致介绍,能够给不同专业和研究领域的研究者提供一些方向性的启发。
3.4 该综述的重要意义 该综述以口腔疾病与氧化应激的关系为基础,介绍了口腔疾病氧化应激的损伤机制和抗氧化纳米材料清除活性氧改善氧化应激的机制,总结了抗氧化纳米材料应用于口腔各类疾病的研究现状、不足及未来的发展前沿,为进一步优化口腔抗氧化纳米材料的性能提供新的策略和思路,并推动高性能、低成本、稳定安全的口腔抗氧化纳米材料进入临床。
3.5 课题专家组对未来的建议 龋病、牙周炎和口腔癌等口腔相关疾病的发病率居高不下,严重影响人类的生活质量[101-102]。近年来的研究均表明氧化应激在口腔疾病发病中起着重要作用,也有不少研究者对内在的生物学机制进行了探索,但是对疾病的完整发展规律探索还不够,同时疾病个体化差异特点也没有得到很好的认识与总结,使得药物的研究和使用并不精准[103]。因此,未来需要花大量的精力更加深刻认识每一种疾病的发病机制和发展规律,将点对面转化为点对点地解决问题。
学科交叉融合能够解决诸多错综复杂的问题,过去近20年中,世界各国都相继发布和实施了纳米科技研究和发展计划,纳米材料正以高速发展的势头涵盖到各个领域。近几年,抗氧化纳米材料与口腔医学的融合为口腔疾病的治疗提供了新的尝试,相关研究也都在动物体层面取得了不错效果,但距离临床转化仍有一段距离,特别是抗氧化纳米材料的潜在生物安全性问题尚未完全解决。未来,研究者需要在对口腔疾病机制和规律更深层次认识的基础上,优化抗氧化纳米材料的性质,以期早日实现临床应用的转化。
龋齿、牙髓炎、牙周炎和口腔癌等口腔疾病发病与氧化应激关系密切,临床研究表明抗氧化治疗辅助传统的治疗能更有助于疾病愈合。相较于天然抗氧化物,抗氧化纳米材料稳定性好,清除效率高,具有广阔的医学应用前景。然而现有研究大多聚焦于抗氧化纳米材料在全身疾病中的应用,尤其是肿瘤和心脑血管疾病,关于口腔疾病的描述较少。因此,该文总结了近十几年抗氧化纳米材料在口腔各类疾病中的研究应用,分析了其临床转化所面临的问题,并根据研究现状和临床应用要求,提出了未来口腔抗氧化纳米材料的研究方向,为实现抗氧化纳米材料的临床转化提供了新思路。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||