中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (22): 4771-4783.doi: 10.12307/2025.446
• 生物材料综述 biomaterial review • 上一篇 下一篇
纳米粒子在骨组织工程化基因修饰治疗中的应用
李光照,裴锡波,王 剑
- 口腔疾病防治全国重点实验室,国家口腔医学中心,国家口腔疾病临床医学研究中心,四川大学华西口腔医院修复科,四川省成都市 610041
-
收稿日期:2024-05-06接受日期:2024-06-13出版日期:2025-08-08发布日期:2024-12-06 -
通讯作者:王剑,博士,教授,口腔疾病防治全国重点实验室,国家口腔医学中心,国家口腔疾病临床医学研究中心,四川大学华西口腔医院修复科,四川省成都市 610041 -
作者简介:李光照,女,1999年生,山西省人,汉族,四川大学华西口腔医(学)院在读硕士。 -
基金资助:国家自然科学基金项目(82271034),项目负责人:王剑;国家自然科学基金项目(82271016),项目负责人:裴锡波;四川省中央引导地方科技发展专项项目 (2023ZYD0109),项目负责人:裴锡波
Application of nanoparticles in gene modification therapy for bone tissue engineering
Li Guangzhao, Pei Xibo, Wang Jian
- State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases, Department of Prosthodontics of West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2024-05-06Accepted:2024-06-13Online:2025-08-08Published:2024-12-06 -
Contact:Wang Jian, MD, Professor, State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases, Department of Prosthodontics of West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Li Guangzhao, Master candidate, State Key Laboratory of Oral Diseases & National Center for Stomatology & National Clinical Research Center for Oral Diseases, Department of Prosthodontics of West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
Supported by:National Natural Science Foundation of China, No. 82271034 (to WJ); National Natural Science Foundation of China, No. 82271016 (to PXB); Fund of Sichuan Provincial Department of Science and Technology, No. 2023ZYD0109 (to PXB)
摘要:
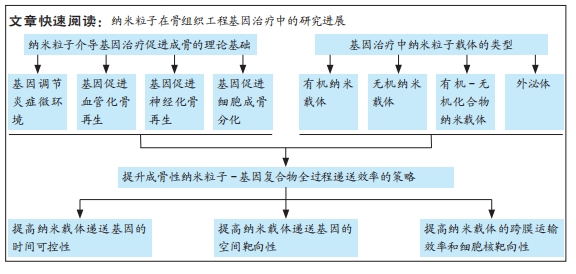
文题释义:
骨组织工程:由细胞、支架和生物活性物质组合构成的人工合成骨移植物,将其植入到体内骨缺损部位可以促进内源性骨再生。纳米粒子载体:粒径为10-1 000 nm的微小粒子,可以由各种材料制备而成,微小的尺寸赋予其很多特殊的性质,非常适合作为载体运送核酸等生物大分子。
背景:传统的骨组织工程技术治疗临界骨缺损存在成骨效率低、安全性差等问题。而以非病毒纳米粒子为基因载体构建的基因强化型骨组织工程移植物,具有更高的成骨效率和安全性,引起了国内外学者的广泛关注和研究。
目的:对当前国内外有关纳米粒子在组织工程成骨基因治疗研究中取得的新技术、新方法以及面临的挑战等进行综述,旨在为纳米粒子介导的骨组织工程基因治疗研究提供参考。方法:第一作者在PubMed、Web of Science和中国知网数据库上进行文献检索,并以“Bone defect repair,Bone tissue engineering,Gene delivery,Nanoparticles,Non-viral gene vector,Sustained release technology,Sequential release,Targeted delivery”作为英文检索词,以“骨缺损修复,骨组织工程,基因递送,纳米粒子,非病毒基因载体,缓释技术,序贯释放,靶向性递送”作为中文检索词,最终纳入84篇文献进行总结。
结果与结论:①在骨缺损愈合的各个生理阶段进行针对性的基因递送可以显著增强骨修复效果。在早期炎症阶段,通过纳米粒子递送抗炎基因来调节炎症反应,可以为后续骨愈合奠定基础;在血管新生期,向局部递送促血管化基因有助于形成高度组织化、可灌注的血管系统,加快骨愈合速度;随着血管化的进行,骨骼的神经再支配也开始发生,此时递送促神经再生的功能性基因有利于促进神经化骨再生;在成骨阶段,通过构建纳米粒子-成骨基因复合物,可以直接提升支架及体内新骨形成的效率。②各种有机、无机纳米颗粒、金属有机框架和外泌体等非病毒纳米载体,在骨组织工程基因治疗中具有巨大的潜力,这些纳米基因载体各有其独特的优势和不足,因此在实际应用时,需要根据基因转染效率、生物安全性和成骨特性等因素选择最合适的类型。③为了全面提升递送基因的效果,目前主要通过对纳米载体进行各种功能设计来增强基因转染效率,包括增强缓释性和多基因递送序贯性等时间调控能力、增强对骨组织和成骨相关细胞的空间靶向能力、增强跨膜运输效率和细胞核靶向能力等全过程调控手段。④未来要进一步推动纳米粒子介导的骨组织工程基因治疗在临床上的应用,还需要克服诸多技术挑战,包括提高有机纳米基因载体的基因转染效率、降低无机纳米载体的生物安全性风险、优化新型纳米载体的生产工艺以及促进其它生理过程与成骨交互作用等,这些问题也是未来骨组织工程基因治疗的研究热点和潮流。
https://orcid.org/0000-0002-7804-3479 (李光照);https://orcid.org/0000-0002-5137-0330 (王剑)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
李光照, 裴锡波, 王 剑. 纳米粒子在骨组织工程化基因修饰治疗中的应用[J]. 中国组织工程研究, 2025, 29(22): 4771-4783.
Li Guangzhao, Pei Xibo, Wang Jian. Application of nanoparticles in gene modification therapy for bone tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4771-4783.
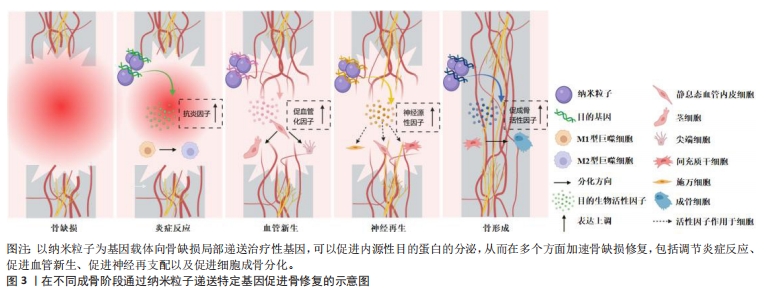
2.1.1 纳米粒子递送基因调节炎症微环境 骨损伤时机体会释放出多种趋化因子和促炎因子,人体处于急性炎症反应期。巨噬细胞在促炎因子的作用下极化为M1表型,进一步释放大量促炎分子来放大炎症反应。这种急性反应通常在骨损伤后24-48 h内达到顶峰,并在7 d内完全消退,此时中性粒细胞分泌抗炎因子使巨噬细胞由促炎M1表型转变为组织修复M2表型,从而启动伤口愈合。然而,对于严重骨损伤或慢性炎症情况下发生的骨缺损,往往会造成炎症因子在初期过度释放,导致急性炎症迟迟不能转入消退过程,造成骨愈合不良甚至失败[6]。因此,在此种情况下的骨缺损早期,及时通过组织工程移植物中的纳米载体引入抗炎基因,以达到促炎与抗炎环境的平衡是十分关键的[7]。微小RNA133a是一种成骨的负向调节因子,CASTANO等[8]使用纳米羟基磷灰石颗粒负载微小RNA133a拮抗基因转染至大鼠间充质干细胞,发现转染后7 d时成骨性基因的表达水平显著高于未转染组。之后他们用胶原支架将该基因-载体复合物递送至大鼠颅骨缺损处,在植入后1周时观察到比对照组更多的钙沉积,4周时新骨体积比空白支架组多2.2倍。同时,他们对不同组别之间的泛巨噬细胞标记CD68+(识别M1型和M2型巨噬细胞)和 CD206+(识别M2型巨噬细胞)进行了定性和定量分析,发现促炎型与促重塑型巨噬细胞的比值与骨修复效果呈现相反的趋势,证明了该基因-载体复合物可以强烈促进CD206+M2型巨噬细胞的募集与激活,进而加速骨修复进程。通过纳米载体递送转化生长因子β基因或白细胞介素1受体拮抗基因也可以调节炎症,为后续的骨修复过程奠定基础[9-10]。
2.1.2 纳米粒子递送基因促进血管化骨再生 在骨缺损发生后的第24-48 h,损伤区域的炎症和低氧状态刺激附近的细胞分泌各种促血管生成因子,激活静息态的内皮细胞分化为尖端细胞或茎细胞,开始血管出芽,同时伴随胶原纤维的沉积,由此开启骨缺损的第二个阶段——纤维血管化期。对于骨组织工程修复材料来说,仅靠周围血管的渗出无法使其获得充足的氧气,为避免坏死,需要及时在移植物内部构建血管系统[11]。已有众多研究通过组织工程支架直接递送血管内皮生长因子、碱性成纤维细胞生长因子和血小板源性生长因子等治疗性蛋白来重建骨血运,但一些临床试验未能证明这样的递送方式有显著的血管化效果,这可能是由于外源性生长因子半衰期短且在体内稳定性差造成的[12]。通过纳米级基因载体来递送成血管基因,可以有效解决上述问题[13-14]。CHAKKA等[15]使用聚乙烯亚胺纳米颗粒将血管内皮生长因子基因转染至人脐静脉内皮细胞,发现基因转染组的血管内皮生长因子蛋白表达长达6 d,骨钙素基因表达水平是未转染组的4倍。使用支架将该基因-载体复合物移植入大鼠颅骨缺损区,发现基因转染组的新生血管数量和新骨形成体积显著增强。也有研究利用纳米载体同时负载了骨形成基因与血管形成基因,实现了成骨与成血管的“双赢”,明显加速了骨缺损愈合的速度和质量[16]。
2.1.3 纳米粒子递送基因促进神经化骨再生 骨缺损不仅会造成局部血管破裂,还会损伤与血管伴行的周围神经组织。在骨愈合过程中,受损的感觉神经和交感神经在血管新生前表达酪氨酸羟化酶、降钙素基因相关肽等早期神经性标志物,并紧随血管化开始神经组织的再生和重塑。血液为神经再生提供了必要的养分支持,而神经再生通过分泌多种神经递质及神经肽等神经源性因子,进一步调节血管生成,并在提供感觉、促进细胞成骨分化及调控骨代谢微环境等方面持续发挥作用,这对于促进功能性骨修复至关重要[17]。WANG等[18]研究发现,在大鼠股骨骨折后3 d时,骨折附近的骨组织内施万细胞分泌的外泌体数量急剧增加,证明神经再生在早期即参与骨修复进程。通过分析分子机制,发现施万细胞的外泌体作为一种天然的纳米级基因载体,富集了大量的微小RNA let-7c-5p,通过靶向Acvr1b基因下调Smad2/3信号通路,从而促进了骨髓间充质干细胞的成骨分化。进一步用支架将外泌体递送至大鼠体内,显著提升了大鼠颅骨缺损的骨修复效果。为了实现神经支配与血流灌注的协同促成骨作用,QIAO等[19]利用聚乙烯亚胺和杂交细胞膜构建了纳米级基因载体,同时负载上调血管内皮生长因子受体表达和上调p75神经营养因子受体表达的微小RNA。他们发现,通过双基因转染可以促进间充质干细胞对成血管相关因子和神经再生相关因子的分泌,从而增强细胞的成骨分化能力,在动物实验中显示出良好的神经血管化骨愈合效果。此外,神经再生与抗炎治疗的耦合对于早期骨修复也具有协同促进作用。LEI等[20]分别利用水凝胶支架和介孔二氧化硅纳米颗粒对阿司匹林和微小RNA 222进行顺序性递送,通过阿司匹林营造早期抗炎环境、随后微小RNA 222促进神经再生,显著提升了大鼠下颌骨缺损的愈合速度。
2.1.4 纳米粒子递送基因促进骨形成 新生血管系统提供的养分会引发一系列成骨反应,其中最重要的反应是间充质干细胞在多种细胞因子的诱导下分化为成骨细胞或软骨细胞,由此进入了骨缺损愈合的最后阶段——骨形成期。骨形态发生蛋白是成骨阶段中代表性的明星分子家族,其中骨形态发生蛋白2,4,6,7和9型具有强大的骨诱导活性。TENKUMO等[21]通过胶原支架将纳米羟基磷灰石-骨形态发生蛋白2基因复合体递送至小鼠体内,引发了更长期、更大量的骨形态发生蛋白2蛋白分泌。胰岛素样生长因子1已被证明有确切的直接促成骨作用,转化生长因子β1不仅在炎症期发挥抗炎作用,在成骨期也可以促进细胞成骨分化和骨基质形成,在组织工程化移植物中通过纳米粒子递送编码这些目的基因的质粒DNA均可以起到良好的促成骨作用[22-23]。除DNA之外,大量具有直接或间接促骨重塑作用的RNA逐渐被发掘,为骨组织工程基因治疗提供了新工具。研究发现,使用信使RNA可能比DNA具有更优异的治疗效果,例如,研究发现用聚乙烯亚胺纳米颗粒负载骨形态发生蛋白2信使RNA,比负载骨形态发生蛋白2质粒DNA对骨髓间充质干细胞的转染效率和促成骨能力更高。
为了减小外源性信使RNA的免疫原性,研究人员进一步利用脂质体纳米粒子同时负载骨形态发生蛋白2信使RNA和免疫逃逸蛋白信使RNA,发现其不仅显著增强了间充质干细胞中骨形态发生蛋白2的产生,而且通过体内支架植入明显加快了小鼠的骨重建[24]。微小RNA已被证明在成骨全过程中可以同时针对多项靶标产生调控作用[25]。研究发现通过纳米粒子递送微小RNA 21、微小RNA 210及微小RNA7b等均可促进动物骨缺损处的血管化骨再生[26-28]。此外,使用小干扰RNA对成骨抑制性基因进行沉默,则提供了一种逆向思维。例如XING等[29]构建了金纳米颗粒-小干扰RNA复合体以靶向抑制成骨细胞中组织蛋白酶K的表达,通过长达8 d的基因缓释有效促进了骨形成。
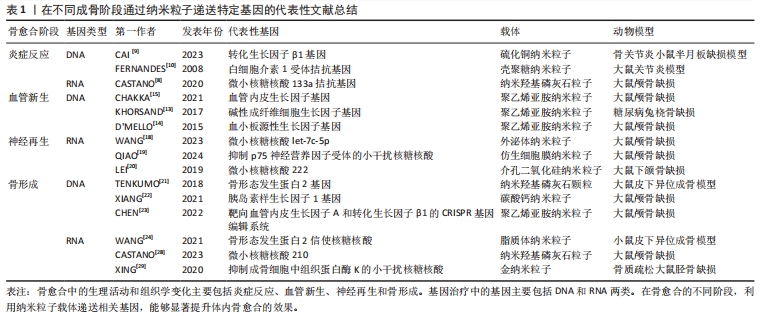
文章总结了不同成骨阶段通过纳米粒子递送特定基因的代表性文献研究进展,见表1,而在不同成骨阶段通过纳米粒子递送特定基因促进骨修复的示意图见图3。
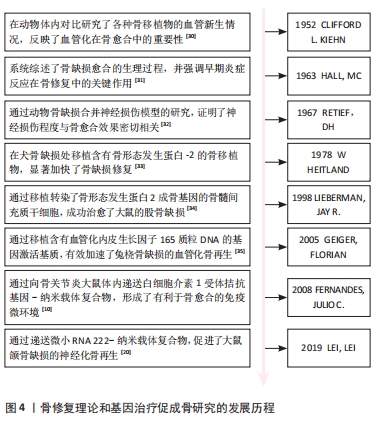
研究人员对骨愈合理论的认知经历了一个逐步发展的过程。在20世纪中期,通过临床观察和动物实验,人们初步认识到血液再灌注在骨愈合中的重要作用[30]。随着分子生物学技术的发展,研究者们开始更深入地研究骨愈合的生理机制。20世纪60年代的研究表明,骨愈合的早期炎症反应及其转归对成骨效果影响深远,且神经组织的再生不足会导致骨愈合延迟或不愈合[31-32]。随后,骨形成期中的经典成骨诱导因子如骨形态发生蛋白2等被分离出来,并得到了快速应用。在1978年,HEITLAND等[33]构建了含有经典骨形态发生蛋白2的骨移植物,成功修复了犬骨缺损。自20世纪90年代末起,组织工程和基因工程技术的发展使研究人员能够通过构建转基因细胞或基因激活支架,从而优化骨移植效果,其中,成骨基因、促血管化基因、炎症调节基因得到了广泛应用[10,34-35]。
21世纪20年代左右,研究人员开始注重神经化骨再生疗法,并据此开发出了神经性转基因骨移植物[20]。具体时间脉络见图4。

2.2 基因治疗纳米粒子的合成方法及类型 骨组织工程基因治疗中的纳米基因载体种类繁多,主要包括有机纳米载体、无机纳米载体、有机-无机化合物纳米载体和外泌体等类型,其合成方法也多种多样,如有机纳米载体的合成方法主要包括乳液聚合法、溶剂挥发法、纳米共沉淀法及离子交联法等[36]。乳液聚合法是指将单体、乳化剂、水和引发剂混合形成乳液,通过聚合反应得到纳米颗粒,这种方法常用于制备聚乙烯亚胺纳米颗粒等人工合成阳离子聚合物。溶剂挥发法通过挥发聚合物溶液中的有机溶剂,使纳米材料固化成为颗粒,适用于制备聚乳酸、聚乳酸-羟基乙酸共聚物等合成聚合物纳米颗粒。纳米共沉淀法是将含有聚合物的有机溶液与水迅速混合,使聚合物在水相中快速沉淀为纳米颗粒。离子交联法适用于制备壳聚糖等天然聚合物纳米载体,通过离子交联剂与多糖溶液中的分子交联形成纳米颗粒。无机纳米载体的制备方法主要有共沉淀法、溶胶-凝胶法及微乳液法等[37-38]。共沉淀法通过将金属盐溶液与沉淀剂混合,调节反应条件使金属离子共沉淀形成纳米颗粒,主要用于合成纳米羟基磷灰石等无机载体。溶胶-凝胶法是将金属有机化合物或金属盐溶解在溶剂中,通过水解和缩合反应形成溶胶,再经过凝胶化过程形成纳米颗粒,适用于制备二氧化硅等氧化物纳米颗粒。微乳液法是指在微乳液体系中,前驱体溶液被限制在微小的液滴中,通过化学反应生成金属纳米颗粒。溶剂热法和常温溶液法是指将金属盐和有机配体溶解在溶剂中,分别在高温高压或常温条件下反应生成金属有机框架纳米粒子。外泌体目前主要通过超速离心法和密度梯度离心法来获取[39]。
2.2.1 有机纳米载体 纳米阳离子脂质体是目前细胞实验中常被用作判断基因转染效率的金标准,因为其简单的脂质双分子层结构不仅易于制备,而且很容易通过与细胞膜相融合而促使目的基因进入细胞内部,具有优秀的基因转染效率和生物相容性[40]。阳离子脂质体的缺陷在于虽然其免疫原性较低,但是在血浆蛋白的干扰下容易降解或聚集,当其过度聚集时会激发免疫应答和细胞毒性。有研究通过制备聚乙二醇化的可电离阳离子脂质体,减少了基因-载体复合物在血浆中受到的干扰,延长其体内循环时间[41]。然而,慢性炎症、肿瘤和代谢紊乱性疾病等特殊状态中的氧化应激、高酶活性和能量代谢异常,可能会破坏脂质体和细胞的结构,影响基因递送效率[40]。过去的研究主要集中在使用脂质体递送目的基因以促进成骨作用。近期的研究发现,用低浓度阳离子脂质体对巨噬细胞和间充质干细胞进行短期处理,可以适度上调促炎因子的表达,从而增强体内早期和晚期成骨作用。此外,通过调控水凝胶支架中阳离子脂质体的释放速率,可以实现对骨再生的选择性免疫调节。这项研究提示,优化基因载体的浓度和作用时间,可以有效加强骨组织工程中基因-载体复合物的促成骨效果[42]。
人工合成阳离子聚合物与基因复合后会形成纳米级多链结构,其优势首先在于通过“质子海绵效应”可以使基因-载体复合物及时逃离溶酶体,其次是可以对其支链上的众多反应性氨基进行官能团化来获得更佳的理化性质。合成阳离子聚合物在成骨方面并无明显作用。研究表明,虽然纳米级聚乙烯亚胺可以上调人间充质干细胞中骨涎蛋白的表达,但对其他成骨细胞外基质标志物的表达没有促进效果;只有当其用作递送成骨性基因等生物活性物质时,才能实现较为理想的骨修复效果[43]。然而,虽然合成阳离子聚合物的高分子量赋予了其强基因携带能力,但是也随之带来了细胞毒性较大的缺陷[44]。目前研究人员主要通过结合利用低分子量聚合物和化学改性手段来实现转染效率与生物相容性之间的平衡。例如WANG等[45]制备了双氨基甲酸交联的低分子量聚乙烯亚胺,通过可降解的乙烯双氨基甲酸酯键将成骨性小干扰RNA递送至骨髓间充质干细胞,获得了比高分子量聚乙烯亚胺更低的细胞毒性和更高的基因转染效率,显著优化了大鼠临界骨缺损的修复效果。
可生物降解聚合物是一种生物相容性和降解性极高的聚合物材料,在降解时经水解或酶解反应转化为单糖、氨基酸及乳酸等小分子,最终进入三羧酸循环或通过肾脏代谢。因此,与脂质体和人工合成阳离子聚合物相比,可生物降解聚合物纳米载体在降低免疫原性和细胞毒性方面具有显著优势。根据来源,可生物降解聚合物分为天然聚合物和合成聚合物。天然可降解聚合物包括多糖、多肽和聚酯,如壳聚糖、透明质酸、丝素、明胶、聚羟基丁酸酯和聚羟基戊酸酯等;合成聚合物主要包括聚乳酸、聚乙醇酸、聚左旋乳酸、聚乳酸-羟基乙酸共聚物等[46]。虽然天然可降解聚合物纳米载体与细胞膜的相互作用和基因压缩能力较弱,但其药物缓释效果通常优于其他有机纳米基因载体。CHEN等[47]使用壳聚糖纳米颗粒负载微小RNA 199a-5p作用于人间充质干细胞,发现转染后第21天释放出65%的基因药物,持续促进成骨分化[41]。合成可降解聚合物纳米载体通过分子设计可以调控其性能和降解速率,达到比天然聚合物更精确和长效的基因释放效果。在成骨性能方面,合成可降解聚合物主要通过调节组织工程支架的表面形貌来改善细胞附着和增殖,但作为纳米基因载体时对成骨的效果并不明显。天然聚合物在一定程度上可以间接辅助骨再生,例如,壳聚糖和丝素可以分别通过抗菌作用和上调抗炎因子表达起到改善成骨环境的作用[48]。
2.2.2 无机纳米载体 纳米羟基磷灰石晶体与天然骨骼的化学组成类似,因此生物相容性很高,而且可以通过缓释钙离子和磷酸根来发挥多种直接和间接成骨作用。例如,研究发现纳米羟基磷灰石在炎症诱导的巨噬细胞内过表达白细胞介素10,通过激活STAT/cMaf/白细胞介素10信号通路促进巨噬细胞向M2型转换,从而为体内骨愈合提供良好的抗炎环境[49]。在血管新生方面,纳米羟基磷灰石可以上调FAK/基质金属蛋白酶和磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase,PI3K)/AKT/一氧化氮合成酶等通路,增强血管内皮生长因子表达,促进内皮细胞黏附和迁移,诱导血管化骨再生。此外,它还可以通过上调骨形态发生蛋白/Smad,FAK/Src,Ras/细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)和ERK/丝裂原活化蛋白激酶(mitogen activated protein kinase,MAPK)等信号通路,促进细胞成骨分化和后期骨重塑[48]。KASAI等[50]使用纳米羟基磷灰石涂层对骨移植物进行表面改性,并将其植入大鼠骨内,结果显示植入后2周时,该植入物显著上调了成骨基因的表达,且其骨整合程度明显高于对照组。然而,应用纳米羟基磷灰石基因载体促进成骨时存在潜在的细胞毒性,这主要与其使用浓度有关,因为过量的钙离子释放可能导致细胞溶酶体破裂,造成氧化应激急剧上升而致细胞凋亡,大量纳米羟基磷灰石的团聚和沉积也会对细胞造成机械损害。
金属纳米颗粒,尤其是金纳米粒子,具有生物惰性高、表面活性大及制备简便等优点。在骨缺损的炎症环境中,金纳米颗粒通过电子交换吸附自由基,减少细胞内、外活性氧的过度产生,并通过调节促炎因子和抗炎因子的表达来介导巨噬细胞从M1型向M2型转化,以抑制过度炎症。另一方面,金纳米粒子可以激活Wnt/β-catenin,MAPK38/ Runt相关转录因子2(Runx2)等信号通路,促进间充质干细胞和原代成骨细胞的成骨分化,还可以通过激活机械刺激反应性Yes相关蛋白促进骨缺损的矿化和新骨形成[48,51]。此外,其成骨效果和细胞毒性与形貌特征显著相关。例如研究发现球状的金纳米粒子比棒状生物相容性更高,直径在50 nm以下的金纳米粒子对细胞的成骨分化刺激作用更强[52-53]。PAN等[54]利用直径为20 nm的球形金纳米粒子将微小RNA29b分别递送至人间充质干细胞和小鼠成骨前体细胞,发现空载金纳米粒子在成骨时长期发挥促细胞分化作用,脂质体负载微小RNA组在早期主要促进细胞成骨分化,在晚期则加强了胶原蛋白的合成和矿化,而金纳米粒子负载微小RNA组展现出了比其他组更低的细胞毒性和更强的体内成骨综合效果。研究表明联合使用具有骨诱导特性的纳米基因载体和成骨调控性目的基因可以发挥二者的协同作用,构建更优越的骨组织工程移植物。此外,金可与其他金属构成合金纳米颗粒或杂化纳米粒子,通过不同金属的协同反应构建更全面的基因载体,例如,金钯纳米粒子因其优异的催化活性、稳定性和光学传感性能,已在生物医学传感中得到广泛应用[55],尽管其在骨组织工程中的应用尚处于探索阶段,但因优秀的综合性能使其有望成为未来基因载体材料的重要候选。
介孔二氧化硅纳米载体降解时释放的硅离子已被证明可以激活经典Wnt/β-catenin通路,促进间充质干细胞成骨分化。有人进一步研究了介孔二氧化硅颗粒的免疫调节作用,发现介孔二氧化硅纳米颗粒释放的硅离子一方面促进了M1型巨噬细胞进行细胞自噬,另一方面减少巨噬细胞抗炎因子的分泌,并通过激活经典Wnt通路来抑制Wnt5A/Ca2+促炎通路,为骨髓间充质干细胞的成骨分化营造了有利的免疫微环境[56]。介孔二氧化硅纳米颗粒的优势还在于具有较高的生物相容性和降解性,而且其表面高度有序的孔隙不仅有利于细胞黏附和增殖,还提供了大量基因载荷和分子修饰空间。YAN等[57]用阳离子聚乙烯亚胺涂覆介孔二氧化硅-微小RNA26a纳米颗粒,使纳米颗粒表面电荷由负转正,从而显著提高了载体与细胞膜之间的静电吸引力。随后,将KALA细胞穿透肽与该复合粒子偶联,进一步增强了骨髓间充质干细胞对微小RNA26a复合颗粒的摄取能力,有效促进了细胞的成骨分化。研究还表明,即使在高浓度下,介孔二氧化硅纳米粒子载体也没有明显的细胞毒性,对细胞活性的不利影响主要由基因转染引起。
以氧化石墨烯、黑磷纳米片为代表的二维无机纳米片载体是指厚度为单层到几层原子厚度的材料,其多功能性在骨组织工程基因治疗中展现出独特的优势。氧化石墨烯纳米片的单层致密蜂窝状结构由碳原子构成,赋予其强大的电、热、光、磁和pH响应能力,大量氧化集团的引入进一步增强了其化学反应活性和高分散性,使其成为靶向肿瘤微环境的出色药物递送载体。在组织工程领域,有研究发现氧化石墨烯可以响应氧化应激信号上调丝/苏氨酸蛋白激酶(serine/threonine protein kinase,AKT)/内皮型一氧化氮合酶/血管内皮生长因子信号通路,从而引发血管新生[58]。另有研究通过表面改性的纳米氧化石墨烯成功将微小RNA29b递送至骨髓间充质干细胞,促进了大鼠颅骨缺损愈合[59]。然而,尚未有研究将其信号响应能力与骨缺损中的细胞内、外微环境特点结合,以提高基因递送效率,这是下一步研究应关注的方向。黑磷纳米片是另一种很有前途的响应性纳米载体,其生物降解性优于氧化石墨烯,且其降解产生的磷酸盐可以与钙离子结合形成磷酸钙,促进原位生物矿化。此外,黑磷纳米片还可以通过上调JAK/STAT/OASL信号通路增强内皮细胞的线粒体功能并抑制炎症反应,促进伤口血管化愈合[60]。有研究利用黑磷纳米片向间充质干细胞递送成骨性小干扰RNA,通过黑磷纳米片载体与基因的协同作用提升了细胞的成骨分化能力[61]。
2.2.3 有机-无机化合物纳米载体 利用化学键使无机纳米颗粒与有机分子结合可以联合发挥二者的优势,全面提升基因载体的性能。金属有机框架是其中代表性的新型纳米基因载体。金属有机框架是由金属无机单元与有机配体连接形成的三维纳米晶体,其高比表面积和密集有序的孔道结构为表面官能团化、内部封装基因提供了广阔空间[62]。金属有机框架的另一优势是在释放核酸药物的同时不断解离出金属离子,这些金属离子对骨重塑起着至关重要的作用。有研究对沸石咪唑框架8纳米颗粒处理的骨髓间充质干细胞进行了基因表达分析,发现纳米颗粒降解时释放的锌离子可以通过激活细胞外信号调节激酶1和细胞外信号调节激酶2蛋白,上调MAPK通路,从而增加成骨相关基因的表达。镁基金属有机框架释放的镁离子不仅能激活成骨细胞中的Notch和PI3K/AKT通路,促进骨髓间充质干细胞成骨分化,还能激活成骨细胞膜上的Ca2+通道,加速局部钙沉积。此外,钴离子和镍离子可以模拟细胞内缺氧条件,刺激血管新生;银离子和铜离子则通过抗菌作用重塑免疫微环境,辅助骨愈合[63]。FENG
等[64]使用沸石咪唑骨架8同时负载了血管化微小RNA21和成骨性微小RNA5106,并通过支架递送至大鼠颅骨缺损处,明显提升了血管化成骨效果。
2.2.4 外泌体 是由细胞自身分泌的纳米级囊泡,具有天然生物学起源的膜结构,比目前大多数有机或无机纳米载体拥有更突出的生物相容性、细胞内化能力和稳定性。在骨组织工程中,通常选用骨相关细胞的外泌体作为基因载体。因为这些外泌体自身含有细胞产生的各种核酸、蛋白质和肽等生物活性物质,并且其表面携带的天然靶向分子能够将这些成骨性分子输送到不同的受体细胞,如间充质干细胞、成骨细胞、软骨细胞、破骨细胞、内皮细胞和免疫细胞。这些外泌体通过旁分泌作用参与炎症调节、氧化应激、血管生成、成骨分化和骨代谢等生理过程,对维持骨稳态有重要的调控作用。因此,工程化的外泌体可以与人工添加的目的基因协同重塑骨新生环境[65]。LIN等[66]利用从软骨雌激素细胞系中分离出的外泌体封装血管内皮生长因子165基因,使用支架将该基因复合物递送至大鼠临界骨缺损处,发现空载组外泌体相对于对照组明显上调了成骨基因表达和新骨形成,而基因-外泌体复合体组通过增加新生血流灌注,进一步加快了骨愈合速度。应用外泌体基因载体的主要限制在于目前制备外泌体的技术复杂,剂量控制和标准化难度大,因此仍需深入研究,以实现大规模生产和确保安全性[39]。
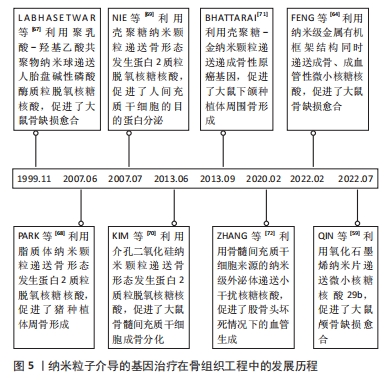
纳米粒子基因载体的性能特点见表2。纳米粒子介导的基因治疗在骨组织工程中的发展历程见图4。

在20世纪90年代末,研究人员开始认识到纳米粒子在基因递送方面的潜力,并成功将纳米载体-基因复合物递送至体内,促进了骨缺损愈合[67]。在2000年代中期至2010年代初,为了提高基因递送效率和生物相容性,科学家们开始对纳米颗粒进行功能化处理,开发出多种表面修饰技术和新型纳米载体类型[68-71]。在2020年代至今,多功能纳米载体如外泌体和金属有机框架等的研发成为热点[59,64,72],这些载体本身具有成骨治疗作用和刺激响应特性,可以与之递送的目的基因协同提升成骨效果,同时实现基因递送的精准靶向,减少不良反应并提高治疗效果。具体时间脉络见图5。

2.3 提升成骨性纳米粒子-基因复合物全过程递送效率的策略
2.3.1 提高纳米载体递送基因的时间缓释性和序贯性 临界骨缺损的愈合是一个由多种信号通路级联交互而成的缓慢进程,这就要求组织工程移植物应持续释放目的基因来长期维持局部药物作用浓度,以提高疗效、降低不良反应,同时应进行双基因或多基因的序贯性递送,以模拟生理状态下不同生长因子的作用顺序,重塑成熟、稳定的骨骼系统。目前实现基因缓释和序贯递送的方法主要包括:构造多层次纳米颗粒、设计刺激响应性纳米载体和使用可调控生物降解性支架等。可生物降解的聚乳酸-羟基乙酸共聚物核壳结构是多层次纳米载体的代表,其内核负责存储基因,外壳通过可控性的解体逐步释放内核,实现基因的缓释。ZHANG等[73]利用纳米级超支化共聚物与微小RNA26a复合形成稳定的多链体,然后封装于聚乳酸-羟基乙酸共聚物微球内形成双时段性递送基因载体。通过改变聚乳酸-羟基乙酸共聚物的分子量可以调控其降解速度,从而缓慢释放多链体,多链体进一步解体释放微小RNA26a,在临界骨缺损的大鼠体内促进了成骨基因的长期表达。核壳结构同样适用于生长因子的序贯递送。WANG等[74]利用同轴针制备了以纳米级聚左旋乳酸-骨形态发生蛋白2复合物为核心,以聚乳酸-羟基乙酸共聚物-碱性成纤维细胞生长因子复合物为外壳的微球。研究发现,在1周内,碱性成纤维细胞生长因子几乎完全释放,而骨形态发生蛋白2则在1个月内逐步释放。研究表明依次递送碱性成纤维细胞生长因子和骨形态发生蛋白2,可显著提升鼠体内的血管化骨再生效果。构建刺激响应性的纳米粒子也可以影响基因释放动力[75]。YAO等[76]构建了具有可见光响应性的胶原-金纳米粒子涂层,发现对其进行光照可以显著加快脂质体-基因复合物的释放速度,通过间隔性光照能够控制其释放速度。通过调节骨组织工程中支架的降解速率,可以改变其内部纳米基因载体的释放速度,进而影响基因的转染时效。水凝胶是一种常用的缓释基质,通过改变交联密度及时间等工艺参数或进行化学修饰,可以调控水凝胶的降解速率[77]。
YANG等[78]发现增加水凝胶交联时间和进行噬菌体肽修饰,可以使水凝胶支架长期缓释纳米载体-基因复合颗粒,增强骨缺损修复效果。进一步联用生物降解性水凝胶支架和微球则可以实现多因子的顺序释放。ZHANG等[79]分别用水凝胶和聚乳酸-羟基乙酸共聚物微球负载了血管内皮生长因子和骨形态发生蛋白2,通过水凝胶的快速降解,首先释放了血管内皮生长因子,然后通过聚乳酸-羟基乙酸共聚物微球的滞后溶胀释放了骨形态发生蛋白2,再用硫酸化壳聚糖修饰水凝胶,进一步延长了二者的缓释时间,最终在体内呈现出良好的血管化骨修复效果。此外,构建新型生物墨水-纳米颗粒复合平台也可以实现基因的差异递送。MONCAL等[80]用壳聚糖纳米颗粒封装骨形态发生蛋白2基因,用β-甘油磷酸盐、壳聚糖溶液和胶原海绵制备的生物墨水封装血小板源性生长因子B基因,构建了血小板源性生长因子B基因初期快速释放、骨形态发生蛋白2基因后期持续释放的序贯递送系统,在体内骨缺损模型中显现出更完善的连续性成骨反应。
提高纳米载体递送基因等货物时间可控性的策略总结见表3。

2.3.2 提高纳米载体至骨相关组织及细胞的空间靶向性 与静脉给药或局部注射药物相比,虽然使用组织工程支架将纳米载体-基因复合物递送至骨缺损区域能够极大提升核酸药物的局部作用浓度,但纳米粒子依然可能会因无序扩散而降低基因转染效率,甚至对非目标组织产生毒性。因此,对纳米载体进行靶向设计以实现对特定细胞的精准定位是十分必要的。对纳米载体进行化学修饰或刺激响应性设计是目前主要的空间靶向手段。
双膦酸盐是一种对骨组织具有极强靶向性的小分子配体。XUE等[81]利用双膦酸盐改性的脂质纳米颗粒递送骨形态发生蛋白2信使RNA,发现双膦酸盐组对后腿和脊柱的基因递送量显著高于无双膦酸盐组,且主要上调了骨髓中细胞的骨形态发生蛋白2表达。肽配体和适配体可以进一步精准定位至成骨相关细胞。肽配体是一种易于生物偶联的低免疫原性短肽。SUN等[82]选择了一段可以与成骨细胞表面骨膜素特异结合的肽配体,将该肽配体与对聚氨酯纳米胶束偶联后,负载了促成骨性小干扰RNA,将该纳米基因复合物与来自骨环境中的细胞进行共孵育,发现成骨细胞中内化的纳米载体数量显著多于其他类型细胞。CH6是一种特异性靶向大鼠和人成骨细胞的适配体,LIANG等[83]将成骨性小干扰RNA封装于CH6改性后的脂质纳米颗粒内,发现CH6主要通过巨胞饮作用促进体外成骨细胞对纳米颗粒的选择性摄取,有效增强了体内成骨细胞的骨形成作用。此外,构建刺激响应性纳米载体也可以达到靶向目的。WANG等[26]发现在电磁场引导下,四氧化三铁纳米粒子-微小RNA21复合物对细胞的转染效率明显高于无磁场组,并且优于标准脂质载体组。该复合物显著提升了骨髓间充质干细胞的成骨分化和人脐静脉内皮细胞的血管化能力。
提高纳米载体至骨相关组织及细胞的空间靶向性策略总结见表4。

2.3.3 提高纳米载体的跨膜运输效率和细胞核靶向性 当纳米载体-基因复合物移动至靶细胞后,首先与细胞表面结合,通过吞噬作用和胞饮作用形成内体进入细胞,然后需要及时从内体逃逸出来以防被溶酶体降解,之后,负载RNA的纳米载体在细胞质中释放目的基因。对于DNA,纳米载体则需将其继续运输至细胞核[41]。细胞穿透肽主要通过与细胞膜和内体膜的相互作用,来促进细胞摄取和内体逃逸。糖胺聚糖结合增强转导肽是一种通过与细胞表面糖胺聚糖相结合来增强跨膜运输的细胞穿透肽,JALAL等[84]用其修饰了封装有骨形态发生蛋白2基因的聚乳酸-羟基乙酸共聚物纳米颗粒,发现修饰组纳米载体在细胞内的基因递送效率是未修饰组的7倍,显著提高了人间充质干细胞的骨形态发生蛋白2蛋白分泌和骨形成能力。核定位序列是由真核细胞核蛋白和病毒蛋白衍生出的短氨基酸序列,该序列具有细胞核入口识别能力,将其与纳米载体相结合,可以提升纳米载体的核靶向性。CHEN等[23]用核定位序列修饰了聚乙烯亚胺纳米颗粒,然后将其用于递送CRISPR基因编辑系统,发现该纳米粒子相比于核定位序列扰乱组,显著增加了其在细胞核内的定位,可有效促进小鼠临界骨缺损的愈合。

| [1] FERRAZ MP. An overview on the big players in bone tissue engineering: biomaterials, scaffolds and cells. Int J Mol Sci. 2024;25(7):3836. [2] RANJBARNEJAD F, KHAZAEI M, SHAHRYARI A, et al. Recent advances in gene therapy for bone tissue engineering. J Tissue Eng Regen Med. 2022;16(12):1121-1137. [3] 王峻峰,谭曼曼,王颖,等.核酸类药物的修饰和递送研究进展[J].浙江大学学报(医学版),2023,52(4):417-428. [4] LI Y, XU C, LEI C. The delivery and activation of growth factors using nanomaterials for bone repair. Pharmaceutics. 2023;15(3):1017. [5] ZHANG LY, BI Q, ZHAO C, et al. Recent advances in biomaterials for the treatment of bone defects. Organogenesis. 2020;16(4):113-125. [6] XIAO B, ADJEI-SOWAH E, BENOIT D. Integrating osteoimmunology and nanoparticle-based drug delivery systems for enhanced fracture healing. Nanomedicine. 2024;56:102727. [7] ZARUBOVA J, HASANI-SADRABADI MM, ARDEHALI R, et al. Immunoengineering strategies to enhance vascularization and tissue regeneration. Adv Drug Deliv Rev. 2022;184:114233. [8] CASTANO IM, RAFTERY RM, CHEN G, et al. Rapid bone repair with the recruitment of CD206(+)M2-like macrophages using non-viral scaffold-mediated miR-133a inhibition of host cells. Acta Biomater. 2020;109:267-279. [9] CAI Y, WU C, OU Q, et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-beta1. Bioact Mater. 2023;19:444-457. [10] FERNANDES JC, WANG H, JREYSSATY C, et al. Bone-protective effects of nonviral gene therapy with folate-chitosan DNA nanoparticle containing interleukin-1 receptor antagonist gene in rats with adjuvant-induced arthritis. Mol Ther. 2008;16(7):1243-1251. [11] JANG HJ, YOON JK. The Role of Vasculature and Angiogenic Strategies in Bone Regeneration. Biomimetics (Basel). 2024;9(2):75. [12] RUST R, GANTNER C, SCHWAB ME. Pro- and antiangiogenic therapies: current status and clinical implications. FASEB J. 2019; 33(1):34-48. [13] KHORSAND B, NICHOLSON N, DO AV, et al. Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J Control Release. 2017;248:53-59. [14] D’MELLO SR, ELANGOVAN S, HONG L, et al. A pilot study evaluating combinatorial and simultaneous delivery of polyethylenimine-plasmid dna complexes encoding for VEGF and PDGF for bone regeneration in calvarial bone defects. Curr Pharm Biotechnol. 2015;16(7):655-660. [15] CHAKKA JL, ACRI T, LAIRD NZ, et al. Polydopamine functionalized VEGF gene-activated 3D printed scaffolds for bone regeneration. Rsc Adv. 2021;11(22):13282-13291. [16] SCHLICKEWEI C, KLATTE TO, WILDERMUTH Y, et al. A bioactive nano-calcium phosphate paste for in-situ transfection of BMP-7 and VEGF-A in a rabbit critical-size bone defect: results of an in vivo study. J Mater Sci Mater Med. 2019;30(2):15. [17] 钟思扬,廖晴,周星宇,等.骨微环境对组织工程骨再生过程的影响[J].中国组织工程研究,2024,28(15):2452-2460. [18] WANG T, LI W, ZHANG Y, et al. Bioprinted constructs that simulate nerve-bone crosstalk to improve microenvironment for bone repair. Bioact Mater. 2023;27:377-393. [19] QIAO F, ZOU Y, BIE B, et al. Dual siRNA-loaded cell membrane functionalized matrix facilitates bone regeneration with angiogenesis and neurogenesis. Small. 2024;20(8):2307062. [20] LEI L, LIU Z, YUAN P, et al. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J Mater Chem B. 2019;7(16):2722-2735. [21] TENKUMO T, VANEGAS SJ, NAKAMURA K, et al. Prolonged release of bone morphogenetic protein-2 in vivo by gene transfection with DNA-functionalized calcium phosphate nanoparticle-loaded collagen scaffolds. Mater Sci Eng C Mater Biol Appl. 2018;92:172-183. [22] XIANG C, TENKUMO T, OGAWA T, et al. Gene transfection achieved by utilizing antibacterial calcium phosphate nanoparticles for enhanced regenerative therapy. Acta Biomater. 2021;119:375-389. [23] CHEN G, DENG S, ZUO M, et al. Non-viral CRISPR activation system targeting VEGF-A and TGF-beta1 for enhanced osteogenesis of pre-osteoblasts implanted with dual-crosslinked hydrogel. Mater Today Bio. 2022;16:100356. [24] WANG P, PERCHE F, MIDOUX P, et al. In Vivo bone tissue induction by freeze-dried collagen-nanohydroxyapatite matrix loaded with BMP-2-NS1 mRNAs lipopolyplexes. J Control Release. 2021;334:188-200. [25] WANG J, CUI Y, LIU H, et al. MicroRNA-loaded biomaterials for osteogenesis. Front Bioeng Biotechnol. 2022;10:952670. [26] WANG T, ZHAO H, JING S, et al. Magnetofection of miR-21 promoted by electromagnetic field and iron oxide nanoparticles via the p38 MAPK pathway contributes to osteogenesis and angiogenesis for intervertebral fusion. J Nanobiotechnology. 2023;21(1):27. [27] DOU C, DING N, LUO F, et al. Graphene-based microRNA transfection blocks preosteoclast fusion to increase bone formation and vascularization. Adv Sci (Weinh). 2018;5(2):1700578. [28] CASTANO IM, RAFTERY RM, CHEN G, et al. Dual scaffold delivery of miR-210 mimic and miR-16 inhibitor enhances angiogenesis and osteogenesis to accelerate bone healing. Acta Biomater. 2023;172: 480-493. [29] XING H, WANG X, XIAO G, et al. Hierarchical assembly of nanostructured coating for siRNA-based dual therapy of bone regeneration and revascularization. Biomaterials. 2020;235:119784. [30] KIEHN CL, CEBUL F, BERG M, et al. A study of the vascularization of experimental bone grafts by means of radioactive phosphorus and the transparent chamber. Ann Surg. 1952;136(3):404-411. [31] HALL MC. Fractures. Can Med Assoc J. 1963;89(6):255-261. [32] RETIEF D H, DREYER C J. Effects of neural damage on the repair of bony defects in the rat. Arch Oral Biol. 1967;12(9):1035-1039. [33] HEITLAND W, VEIHELMANN D, NEUGEBAUER W, et al. Elimination of bone defects with the aid of a transplant (bone morphogenetic protein BMP) prepared according to Urist’s method. Animal experimenst compared with Kieler spongiosa. Chir Forum Exp Klin Forsch. 1978: 251-252. [34] LIEBERMAN JR, LE LQ, WU L, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16(3): 330-339. [35] GEIGER F, BERTRAM H, BERGER I, et al. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20(11):2028-2035. [36] BHARADWAZ A, JAYASURIYA AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698. [37] ZHU L, LUO D, LIU Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int J Oral Sci. 2020;12(1):6. [38] DAMANI M, JADHAV M, JOSHI R, et al. Advances in gold nanoparticles: synthesis, functionalization strategies, and theranostic applications in cancer. Crit Rev Ther Drug Carrier Syst. 2024;41(6):1-56. [39] KIM H I, PARK J, ZHU Y, et al. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp Mol Med. 2024;56(4):836-849. [40] KASHAPOV R, IBRAGIMOVA A, PAVLOV R, et al. Nanocarriers for biomedicine: from lipid formulations to inorganic and hybrid nanoparticles. Int J Mol Sci. 2021;22(13):7055. [41] QADIR A, GAO Y, SURYAJI P, et al. Non-viral delivery system and targeted bone disease therapy. Int J Mol Sci. 2019;20(3):565. [42] JAHANMARD F, KHODAEI A, FLAPPER J, et al. Osteoimmunomodulatory GelMA/liposome coatings to promote bone regeneration of orthopedic implants. J Control Release. 2023;358:667-680. [43] KIM HJ, CHO HB, LEE S, et al. Strategies for accelerating osteogenesis through nanoparticle-based DNA/mitochondrial damage repair. Theranostics. 2022;12(14):6409-6421. [44] ABBASI R, SHINEH G, MOBARAKI M, et al. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: a review. J Nanopart Res. 2023;25(3):43. [45] WANG C, YUAN W, XIAO F, et al. Biscarbamate cross-linked low-molecular-weight polyethylenimine for delivering anti-chordin siRNA into human mesenchymal stem cells for improving bone regeneration. Front Pharmacol. 2017;8:572. [46] SHARMA S, SUDHAKARA P, SINGH J, et al. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers (Basel). 2021; 13(16):2623. [47] CHEN X, GU S, CHEN BF, et al. Nanoparticle delivery of stable miR-199a-5p agomir improves the osteogenesis of human mesenchymal stem cells via the HIF1a pathway. Biomaterials. 2015;53:239-250. [48] BOZORGI A, KHAZAEI M, SOLEIMANI M, et al. Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater Sci. 2021;9(13):4541-4567. [49] MOFARRAH M, JAFARI-GHARABAGHLOU D, FARHOUDI-SEFIDAN-JADID M, et al. Potential application of inorganic nano-materials in modulation of macrophage function: Possible application in bone tissue engineering. Heliyon. 2023;9(6):16309. [50] KASAI H, BERGAMO ET, BALDERRAMA ÍD, et al. The effect of nano hydroxyapatite coating implant surfaces on gene expression and osseointegration. Med Oral Patol Oral Cir Bucal. 2023;22:26303. [51] ZHOU J, ZHANG Y, LI L, et al. Human beta-defensin 3-combined gold nanoparticles for enhancement of osteogenic differentiation of human periodontal ligament cells in inflammatory microenvironments. Int J Nanomedicine. 2018;13:555-567. [52] LI J, LI JJ, ZHANG J, et al. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016;8(15):7992-8007. [53] ZHANG Y, KONG N, ZHANG Y, et al. Size-dependent effects of gold nanoparticles on osteogenic differentiation of human periodontal ligament progenitor cells. Theranostics. 2017;7(5):1214-1224. [54] PAN T, SONG W, GAO H, et al. miR-29b-loaded gold nanoparticles targeting to the endoplasmic reticulum for synergistic promotion of osteogenic differentiation. ACS Appl Mater Interfaces. 2016;8(30): 19217-19227. [55] MADANI-NEJAD E, SHOKROLLAHI A, SHAHDOST-FARD F. Lemon-based core-shell Au@Pd nanoparticles as green nanoprobe: towards fast and visual detection of trifluoperazine. Microchem J. 2024;199:109966. [56] CHEN Z, HAN S, SHI M, et al. Immunomodulatory effects of mesoporous silica nanoparticles on osteogenesis: From nanoimmunotoxicity to nanoimmunotherapy. Applied Materials Today. 2018;10:184-193. [57] YAN J, LU X, ZHU X, et al. Effects of miR-26a on osteogenic differentiation of bone marrow mesenchymal stem cells by a mesoporous silica nanoparticle -PEI - peptide system. Int J Nanomedicine. 2020;15:497-511. [58] HOSEINI-GHAHFAROKHI M, MIRKIANI S, MOZAFFARI N, et al. Applications of graphene and graphene oxide in smart drug/gene delivery: is the world still flat? Int J Nanomedicine. 2020;15:9469-9496. [59] QIN H, JI Y, LI G, et al. MicroRNA-29b/graphene oxide-polyethyleneglycol-polyethylenimine complex incorporated within chitosan hydrogel promotes osteogenesis. Front Chem. 2022;10: 958561. [60] BAI X, WANG R, HU X, et al. Two-dimensional biodegradable black phosphorus nanosheets promote large full-thickness wound healing through in situ regeneration therapy. ACS Nano. 2024;18(4):3553-3574. [61] LI Z, KANG M, XU C, et al. Black phosphorus-based dynamic self-healing hydrogel to integrate demineralized bone matrix and noggin-targeting siRNA for synergistic osteogenesis. ACS Appl Mater Interfaces. 2024. doi: 10.1021/acsami.4c01324. [62] SHYNGYS M, REN J, LIANG X, et al. Metal-organic framework (mof)-based biomaterials for tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2021;9:603608. [63] ZHENG W, MENG Z, ZHU Z, et al. Metal-organic framework-based nanomaterials for regulation of the osteogenic microenvironment. Small. 2024. doi: 10.1002/smll.202310622. [64] FENG H, LI Z, XIE W, et al. Delivery of therapeutic miRNAs using nanoscale zeolitic imidazolate framework for accelerating vascularized bone regeneration. Chem Eng J. 2022;430:132867. [65] HUANG J, XU Y, WANG Y, et al. Advances in the study of exosomes as drug delivery systems for bone-related diseases. Pharmaceutics. 2023;15(1):220. [66] LIN T, ZHA Y, ZHANG X, et al. Gene-activated engineered exosome directs osteoblastic differentiation of progenitor cells and induces vascularized osteogenesis in situ. Chem Eng J. 2020;400:125939. [67] LABHASETWAR V, BONADIO J, GOLDSTEIN SA, et al. Gene transfection using biodegradable nanospheres: results in tissue culture and a rat osteotomy model. Colloids Surf B Biointerfaces. 1999;16(1-4):281-290. [68] PARK J, LUTZ R, FELSZEGHY E, et al. The effect on bone regeneration of a liposomal vector to deliver BMP-2 gene to bone grafts in peri-implant bone defects. Biomaterials. 2007;28(17):2772-2782. [69] NIE H, WANG C. Fabrication and characterization of PLGA-HAp scaffolds for delivery of BMP-2 plasmid composite DNA. J Control Release. 2007;120(1-2):111-121. [70] KIM T, KIM M, ELTOHAMY M, et al. Efficacy of mesoporous silica nanoparticles in delivering BMP-2 plasmid DNA for in vitro osteogenic stimulation of mesenchymal stem cells. J Biomed Mater Res A. 2013; 101(6):1651-1660. [71] BHATTARAI G, LEE YH, LEE MH, et al. Gene delivery of c-myb increases bone formation surrounding oral implants. J Dent Res. 2013;92(9):840-845. [72] ZHANG C, SU Y, DING H, et al. Mesenchymal stem cells-derived and siRNAs-encapsulated exosomes inhibit osteonecrosis of the femoral head. J Cell Mol Med. 2020;24(17):9605-9612. [73] ZHANG X, LI Y, CHEN YE, et al. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat Commun. 2016;7:10376. [74] WANG S, JU W, SHANG P, et al. Core-shell microspheres delivering FGF-2 and BMP-2 in different release patterns for bone regeneration. J Mater Chem B. 2015;3(9):1907-1920. [75] GONG Z, PENG S, CAO J, et al. Advances in the variations and biomedical applications of stimuli-responsive nanodrug delivery systems. Nanotechnology. 2024;35(13):132001. [76] YAO L, WANG X, WENG W, et al. Bioactive nanocomposite coatings under visible light illumination promoted surface-mediated gene delivery. Biomater Sci. 2020;8(13):3685-3696. [77] SU C, LIN D, HUANG X, et al. Developing hydrogels for gene therapy and tissue engineering. J Nanobiotechnology. 2024;22(1):182. [78] YANG Z, LI X, GAN X, et al. Hydrogel armed with BMP-2 mRNA-enriched exosomes enhances bone regeneration. J Nanobiotechnology. 2023;21(1):119. [79] ZHANG S, CHEN J, YU Y, et al. Accelerated bone regenerative efficiency by regulating sequential release of BMP-2 and VEGF and synergism with sulfated chitosan. ACS Biomater Sci Eng. 2019; 5(4):1944-1955. [80] MONCAL KK, TIGLI AR, GODZIK KP, et al. Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats. Biomaterials. 2022;281:121333. [81] XUE L, GONG N, SHEPHERD SJ, et al. Rational design of bisphosphonate lipid-like materials for mRNA delivery to the bone microenvironment. J Am Chem Soc. 2022;144(22):9926-9937. [82] SUN Y, YE X, CAI M, et al. Osteoblast-targeting-peptide modified nanoparticle for siRNA-microRNA delivery. ACS Nano. 2016;10(6): 5759-5768. [83] LIANG C, GUO B, WU H, et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat Med. 2015;21(3):288-294. [84] JALAL AR, DIXON JE. Efficient delivery of transducing polymer nanoparticles for gene-mediated induction of osteogenesis for bone regeneration. Front Bioeng Biotechnol. 2020;8:849. |
| [1] | 李 帅, 刘 桦, 商永慧, 刘义琮, 赵启航, 刘 文. 安氏Ⅱ类错牙合畸形佩戴Twin-block矫治器时上颌骨的应力分布[J]. 中国组织工程研究, 2025, 29(5): 881-887. |
| [2] | 肖 放, 黄 雷, 王 琳. 磁性纳米材料与磁场效应加速骨损伤修复[J]. 中国组织工程研究, 2025, 29(4): 827-838. |
| [3] | 李明哲, 叶翔凌, 王 冰, 余 翔. 负载纳米钽的液晶显示光固化聚乳酸支架制备及促成骨性能[J]. 中国组织工程研究, 2025, 29(4): 670-677. |
| [4] | 余双奇, 丁 凡, 万 松, 陈 伟, 张学俊, 陈 东, 李 强, 林作丽. PLGA/赖氨酸接枝氧化石墨烯纳米粒子复合支架对MC3T3细胞成骨分化的影响[J]. 中国组织工程研究, 2025, 29(4): 707-712. |
| [5] | 张晓宇, 韦善文, 方佳炜, 倪 莉. 普鲁士蓝纳米粒子抗氧化恢复退变髓核细胞线粒体功能[J]. 中国组织工程研究, 2025, 29(34): 7318-7325. |
| [6] | 邓光慧, 向 炜, 苏其帆, 陈小鱼, 王亮为, 万志宏, 吴佳奇, 陈孝均. 骨质疏松性股骨髁骨缺损兔模型制备及其临界值[J]. 中国组织工程研究, 2025, 29(30): 6426-6433. |
| [7] | 李树源, 杨达文, 曾展鹏, 蔡群斌, 张景涛, 周琦石. 诱导膜技术在临界尺寸骨缺损修复中的应用:优势与未来发展[J]. 中国组织工程研究, 2025, 29(28): 6083-6093. |
| [8] | 刘 阳, 杨吉垒, 王文丽, 崔颖颖, 孙琦昊, 李友瑞. 温度响应性水凝胶在骨组织工程中的应用特征[J]. 中国组织工程研究, 2025, 29(28): 6094-6100. |
| [9] | 李泽铭, 张云涛, 王茂林, 侯玉东. 缺氧诱导因子1α调节骨稳态在口腔颌面部疾病治疗中的作用与机制[J]. 中国组织工程研究, 2025, 29(26): 5680-5687. |
| [10] | 俞 磊, 张 巍, 秦 毅, 葛高然, 柏家祥, 耿德春. 贻贝启发接枝骨形态发生蛋白2成骨活性肽的介孔生物玻璃修复股骨髁缺损[J]. 中国组织工程研究, 2025, 29(22): 4629-4638. |
| [11] | 魏鹤翔, 孙 彬, 刘 昊, 刘含强, 夏 鹏. 神经生长因子对骨骼形成及骨疾病的影响[J]. 中国组织工程研究, 2025, 29(20): 4266-4275. |
| [12] | 田家宇, 李多华, 张 锋, 冯 虎, 孙 伟. 构建聚己内酯/纳米金刚石-磷脂复合材料及性能评价[J]. 中国组织工程研究, 2025, 29(16): 3380-3387. |
| [13] | 赵 越, 许 燕, 周建平, 张旭婧, 陈宇彤, 靳正阳, 印治涛. 骨组织工程中传统与仿生支架结构设计的差异[J]. 中国组织工程研究, 2025, 29(16): 3458-3468. |
| [14] | 陈家瀚, 奉 超, 黄晓夏, 牛明慧, 王 鑫, 滕 勇. 骨组织工程研究中的二维黑磷材料[J]. 中国组织工程研究, 2025, 29(10): 2124-2131. |
| [15] | 吴尧昆, 刘成林, 付佳豪, 宋 伟, 陈 浩, 席洪钟, 刘 锌, 杜 斌, 孙光权. 中药有效成分结合骨组织工程材料用于骨修复[J]. 中国组织工程研究, 2025, 29(10): 2141-2150. |
面对上述挑战,基因工程技术与组织工程骨移植物的结合在20世纪90年代中后期应势而生。基因工程技术通过将特定基因加载至种子细胞内,使其以适量、可持续的形式分泌内源性治疗分子并作用于局部组织,不仅提高了成骨效果,而且避免了以往骨组织工程中过量整合外源性蛋白造成的致瘤和免疫风险[2]。骨组织工程中的基因类药物主要分为脱氧核糖核酸(deoxyribonucleic acid,DNA)和核糖核酸(ribonucleic acid,RNA)两类,虽然这些核酸药物具有从基因水平上调控成骨的显著优势,但是人体内的各种核酸酶很容易将裸露的基因降解,而且单一的基因药物缺乏对目标组织的靶向性,容易在体内递送时流失,甚至引起不良反应。为了解决上述问题,研究人员试图通过对目的基因的磷酸基团和核糖进行修饰或修改碱基序列,从而增强基因稳定性和成骨靶向性。但是单一的化学修饰并不能克服体内诸多障碍,而且技术难度高,效果并不稳定[3]。现今,最普遍的基因递送手段是使用基因载体包裹目的基因来隔绝体内核酸酶的破坏作用,同时基因载体易于进行化学改性,可以提升基因-载体复合物的稳定性和对目标组织的靶向能力。
在基因载体中,相比于病毒载体,非病毒载体具有安全性高、修饰方法多及导入方式便捷等优点,具有广阔的应用前景,其中,以纳米粒子为代表的非病毒载体展现出巨大的研究潜力。纳米级基因载体主要有以下3方面优越性[4]:①纳米粒子的尺寸与生物大分子(肽段、蛋白质和核酸等)以及天然骨的组成部分(羟基磷灰石晶体)相近,因此更有利于被细胞内吞和亲和骨组织;②纳米粒子能够与目的基因强效复合,并在血液循环中长时间保持稳定,保证基因药物的生物利用度;③得益于其高比表面积,纳米粒子通过共价结合以及逐层组装,可携带大量表面官能团,因此可以灵活地调控其物理和化学性质,进一步响应于时空调控信号,引发更有序的成骨反应。因此,纳米载体成为骨组织工程基因治疗中亟待深入探索的新兴领域。
以往的综述多围绕纳米粒子在合成骨再生材料中的应用展开,或集中探讨某种纳米粒子介导的多种药物给药方法。而文章旨在聚焦于探讨以纳米粒子为基因载体的基因强化型骨组织工程移植物,通过梳理其在修复骨缺损进程中的治疗作用,进一步综述纳米粒子基因载体的类型、特点及全面提升其基因转染效率的手段,以期为未来纳米粒子介导的骨组织工程基因治疗研究提供参考和
启示。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
1.1.1 检索人及检索时间 第一作者于2024年6月进行检索。
1.1.2 检索文献时限 1952年1月至2024年6月。
1.1.3 检索数据库 中国知网、PubMed和Web of Science数据库。
1.1.4 检索词 英文检索词:“Bone defect repair,Bone tissue engineering,Gene delivery,Nanoparticles,Non-viral gene vector,Sustained release technology,Sequential release,Targeted delivery”。中文检索词:“骨缺损修复,骨组织工程,基因递送,纳米粒子,非病毒基因载体,缓释技术,序贯释放,靶向性递送”。
1.1.5 检索文献类型 综述、研究性原著及临床研究等。
1.1.6 手工检索情况 无。
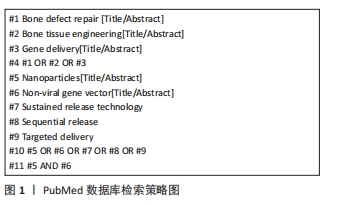
1.1.7 检索策略 以PubMed数据库检索策略为例,见图1。
1.2 入组标准
1.2.1 纳入标准 ①探讨骨组织工程或基因治疗最新进展的综述文章;②涉及纳米粒子介导基因治疗促进成骨的研究原著;③有关骨组织工程移植物或基因治疗实际应用的临床研究。
1.2.2 排除标准 ①研究目的相关性弱的文献;②实验逻辑不清晰、理论陈旧的研究文献。
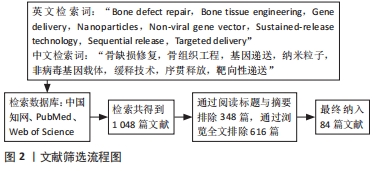
1.3 文献质量评估和数据提取 首先根据关键词初次检索到相关文献1 048篇,根据快速阅读标题和摘要排除与该综述研究目的相关性差的文献348篇,然后通过浏览全文,排除研究内容重复、实验说服力弱的文献616篇,最终筛选出84篇研究手段新颖、理论前沿的文章进行综述,其中中文2篇,英文82篇,见图2。
同时,有机金属框架、外泌体等材料的兴起为构建更加优异的综合性纳米基因载体带来了希望。然而尽管目前的研究已证实这些新型纳米可以有效递送基因进入细胞,但对其具体的递送机制和特点仍了解不足。例如,外泌体载体可以通过内吞作用、膜融合及细胞膜穿透等多种方式被细胞摄取,但对于这些摄取方式如何与骨缺损中的细胞类型和微环境相互作用,以及这些入胞方式如何影响基因货物的释放效率等方面尚缺乏深入研究。这些因素可能直接影响外泌体载体递送基因效果的稳定性。金属有机框架主要通过内吞作用进入细胞,并通过pH响应机制实现溶酶体逃逸。然而,目前还没有研究探究其在溶酶体内释放基因的同时是否促进了溶酶体降解,这与基因递送至细胞质内的效率和最终促进骨愈合的能力密切相关。另一方面,这些新型纳米基因载体的生产技术和质量标准化控制尚不成熟,限制了其大规模应用。例如,外泌体的生产需要严格的细胞培养条件和分离纯化工艺,其大小、形状、含量及纯度等控制和表征难度大,且受到技术及设备等多方面影响,因此其生产质量有待提高。此外,目前的研究主要集中于利用纳米粒子载体-基因复合物进行单一的基因激活或沉默,但对各种基因和纳米粒子在骨愈合中的交互作用尚不明了。进一步深入研究这些复合物在促进骨愈合中的机制,将有助于提升其应用效果和安全性。
3.2 该综述区别于他人他篇的特点 目前已有一些综述对骨组织工程基因治疗中的目的基因和非病毒载体类型进行了总结,但并未对骨愈合全程基因治疗思路进行系统梳理,且未聚焦于非病毒纳米粒子。而文章则以骨愈合的生理过程为线索,综述了骨愈合每一个阶段内纳米粒子递送特定基因促进骨愈合的技术和方法,并对经典和新兴的促成骨性纳米载体进行了详细的特点分析,全面总结梳理了当前全过程提升纳米粒子时空靶向性递送等基因转染效率的前沿策略。该综述为纳米粒子在骨组织工程基因治疗中的应用提供了参考。
3.3 综述的局限性 近年来,随着分子生物学和基因组学的快速发展,CRISPR/Cas9基因编辑系统在骨组织工程基因治疗领域中的应用受到了极大的推动和关注。与传统的基因疗法相比,CRISPR/Cas9系统可以实现更精确的靶基因插入、敲除和编辑,因此其治疗成本更低、效率更高。目前的研究正在探索利用非病毒纳米颗粒递送CRISPR/Cas9系统促进成骨的潜力,并已在动物模型内实现了较好的骨修复效果。但目前CRISPR/Cas9系统在骨组织工程中的应用仍较为单一,其临床转化过程仍存在诸多挑战,由于篇幅所限,文章未能对其进行进一步的深入探讨。
3.4 综述的重要意义 临界骨缺损带来的一系列个人和社会负担已成为困扰国际社会的公共卫生难题,以纳米粒子为媒介构建的基因强化型骨组织工程移植物为解决这一难题带来了新的希望。针对这一新技术,文章聚焦于以骨愈合机制为靶点的目的基因递送,通过深入浅出地阐释其治疗机制,对目前繁杂的骨组织工程基因治疗研究进行了梳理分类,同时从多角度分析了最具代表性的几种纳米粒子载体,最后对近年来提升全过程基因递送效率的各种新兴设计手段进行了总结。文章对下一步纳米粒子介导的基因治疗促进成骨研究具有重要的指导意义。
3.5 课题专家组对未来的建议 尽管纳米粒子介导的组织工程成骨治疗已展现出优良的修复效果,但其临床应用仍面临诸多挑战。未来的研究应该继续探索和创新有机纳米基因载体的合成与表面修饰技术,以提高其稳定性和基因转染效率。此外,需重点关注无机纳米载体在体内骨修复过程中的安全性和有效性,并优化大规模、高质量新型纳米载体的生产技术,从而构建多元化的基因载体。在此基础上,深入研究基因和纳米粒子在骨愈合中的交互作用,以及成骨过程与其他生理反应(如脂肪生成)之间的相互作用机制,以进一步提高骨组织工程基因治疗的效果。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
 #br#
#br#
文题释义:
骨组织工程:由细胞、支架和生物活性物质组合构成的人工合成骨移植物,将其植入到体内骨缺损部位可以促进内源性骨再生。纳米粒子载体:粒径为10-1 000 nm的微小粒子,可以由各种材料制备而成,微小的尺寸赋予其很多特殊的性质,非常适合作为载体运送核酸等生物大分子。
基因载体:用于将目的基因片段引入受体细胞的载体工具,具有保护和运送基因的功能,主要由病毒或非病毒材料构成。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||