[1] YAO Q, WU X, TAO C, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023; 8(1):56.
[2] GU YT, CHEN J, MENG ZL, et al. Research progress on osteoarthritis treatment mechanisms. Biomed Pharmacother. 2017;93:1246-1252.
[3] QIAO X, YAN L, FENG Y, et al. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis. BMC Musculoskelet Disord. 2023;24(1):926.
[4] AMIRI MA, FARSHIDFAR N, MIRON RJ, et al. The Potential Therapeutic Effects of Platelet-Derived Biomaterials on Osteoporosis: A Comprehensive Review of Current Evidence. Int J Biomater. 2023;2023: 9980349.
[5] SHAHID M, KUNDRA R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev. 2017;2(1):28-34.
[6] EVERTS P, ONISHI K, JAYARAM P, et al. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020;21(20):7794.
[7] LV X, ZHAO T, DAI Y, et al. New insights into the interplay between autophagy and cartilage degeneration in osteoarthritis. Front Cell Dev Biol. 2022;10:1089668.
[8] MOUSSA M, LAJEUNESSE D, HILAL G, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146-156.
[9] 屈一鸣,邵高海,张铭华,等.自体富血小板血浆对骨关节炎模型兔软骨细胞凋亡及NLRP3/IL-1β通路的影响[J].中国病理生理杂志, 2020,36(6):1089-1095.
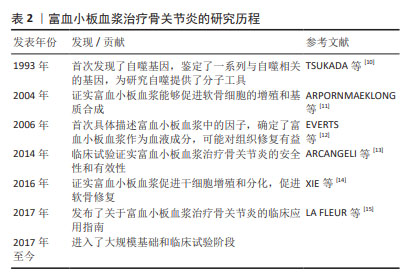
[10] TSUKADA M, OHSUMI Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993; 333(1-2):169-174.
[11] ARPORNMAEKLONG P, KOCHEL M, DEPPRICH R,et al. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004;33(1):60-70.
[12] EVERTS PA, KNAPE JT, WEIBRICH G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
[13] ARCANGELI ML, BARDIN F, FRONTERA V, et al. Function of Jam-B/Jam-C interaction in homing and mobilization of human and mouse hematopoietic stem and progenitor cells. Stem Cells. 2014;32(4):1043-1054.
[14] XIE Q, MA Q, JI B, et al. Incremental value of SPECT/CT in detection of Meckel’s diverticulum in a 10-year-old child. Springerplus. 2016; 5(1):1270.
[15] LA FLEUR P, ARGÁEZ C. Platelet-Rich Plasma Injections for Wound Healing and Tissue Rejuvenation: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2017.
[16] DIKIC I, ELAZAR Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349-364.
[17] YANG F, HU H, YIN W, et al. Autophagy Is Independent of the Chondroprotection Induced by Platelet-Rich Plasma Releasate. Biomed Res Int. 2018;2018:9726703.
[18] HUANG Z, WANG Q, ZHANG T, et al. Hyper-activated platelet lysates prevent glucocorticoid-associated femoral head necrosis by regulating autophagy. Biomed Pharmacother. 2021;139:111711.
[19] DONG C, HUI P, WU Z, et al. CircRNA LOC729852 promotes bladder cancer progression by regulating macrophage polarization and recruitment via the miR-769-5p/IL-10 axis. J Cell Mol Med. 2024; 28(7):e18225.
[20] SHAFIK NM, EL-ESAWY RO, MOHAMED DA, et al. Regenerative effects of glycyrrhizin and/or platelet rich plasma on type-II collagen induced arthritis: Targeting autophay machinery markers, inflammation and oxidative stress. Arch Biochem Biophys. 2019;675:108095.
[21] SHEELA S, ALGHALBAN FM, KHALIL KA, et al. Synthesis and Biocompatibility Evaluation of PCL Electrospun Membranes Coated with MTA/HA for Potential Application in Dental Pulp Capping. Polymers (Basel). 2022;14(22):4862.
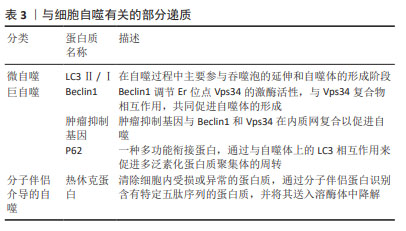
[22] JEONG SJ, ZHANG X, RODRIGUEZ-VELEZ A, et al. p62/SQSTM1 and Selective Autophagy in Cardiometabolic Diseases. Antioxid Redox Signal. 2019;31(6):458-471.
[23] LI L, SHEN C, NAKAMURA E, et al. SQSTM1 is a pathogenic target of 5q copy number gains in kidney cancer. Cancer Cell. 2013;24(6): 738-750.
[24] LI H, MIAO D, ZHU Q, et al. MicroRNA-17-5p contributes to osteoarthritis progression by binding p62/SQSTM1. Exp Ther Med. 2018;15(2):1789-1794.
[25] CHEN N, LI M, YANG J, et al. Slow-sculpting graphene oxide/alginate gel loaded with platelet-rich plasma to promote wound healing in rats. Front Bioeng Biotechnol. 2024;12:1334087.
[26] ZHANG X, CHEN S, SONG L, et al. MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy. 2014;10(4):588-602.
[27] LERNER C, BITTO A, PULLIAM D, et al. Reduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblasts. Aging Cell. 2013; 12(6):966-977.
[28] ZUO D, SUBJECK J, WANG XY. Unfolding the Role of Large Heat Shock Proteins: New Insights and Therapeutic Implications. Front Immunol. 2016;7:75.
[29] SHENDE P, BHANDARKAR S, PRABHAKAR B. Heat Shock Proteins and their Protective Roles in Stem Cell Biology. Stem Cell Rev Rep. 2019;15(5):637-651.
[30] CHEN J, LI C, WANG S. Periodic heat shock accelerated the chondrogenic differentiation of human mesenchymal stem cells in pellet culture. PLoS One. 2014;9(3):e91561.
[31] GIUNTA S, CASTORINA A, MARZAGALLI R, et al. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int J Mol Sci. 2015;16(3): 5922-5944.
[32] BROCKMUELLER A, BUHRMANN C, SHAYAN P, et al. Calebin A modulates inflammatory and autophagy signals for the prevention and treatment of osteoarthritis. Front Immunol. 2024;15:1363947.
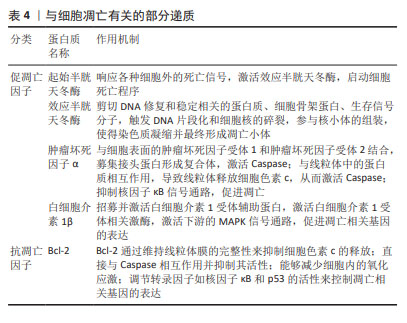
[33] MUSUMECI G, CASTROGIOVANNI P, LORETO C, et al. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: a morphological study. Int J Mol Sci. 2013;14(8):15767-15784.
[34] DING K, SONG C, HU H, et al. The Role of NLRP3 Inflammasome in Diabetic Cardiomyopathy and Its Therapeutic Implications. Oxid Med Cell Longev. 2022;2022:3790721.
[35] WU ZS, LUO HL, CHUANG YC, et al. Platelet Lysate Therapy Attenuates Hypoxia Induced Apoptosis in Human Uroepithelial SV-HUC-1 Cells through Regulating the Oxidative Stress and Mitochondrial-Mediated Intrinsic Apoptotic Pathway. Biomedicines. 2023;11(3):935.
[36] PATEL S, MISHRA NP, CHOUHAN DK, et al. Chondroprotective effects of multiple PRP injections in osteoarthritis by apoptosis regulation and increased aggrecan synthesis- Immunohistochemistry based Guinea pig study. J Clin Orthop Trauma. 2022;25:101762.
[37] KOMURA M, WANG C, ITO S, et al. Simultaneous Expression of CD70 and POSTN in Cancer-Associated Fibroblasts Predicts Worse Survival of Colorectal Cancer Patients. Int J Mol Sci. 2024;25(5):2537.
[38] BUCKLAND J. Osteoarthritis: positive feedback between ADAMTS-7 and TNF in OA. Nat Rev Rheumatol. 2013;9(10):566.
[39] MUKHERJEE A, DAS B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater Biosyst. 2024;13:100090.
[40] UMOH IO, DOS REIS HJ, DE OLIVEIRA ACP. Molecular Mechanisms Linking Osteoarthritis and Alzheimer’s Disease: Shared Pathways, Mechanisms and Breakthrough Prospects. Int J Mol Sci. 2024;25(5): 3044.
[41] YIN WJ, XU HT, SHENG JG, et al. Advantages of Pure Platelet-Rich Plasma Compared with Leukocyte- and Platelet-Rich Plasma in Treating Rabbit Knee Osteoarthritis. Med Sci Monit. 2016;22: 1280-1290.
[42] SAIF DS, HEGAZY NN, ZAHRAN ES. Evaluating the Efficacy of Intra-articular Injections of Platelet Rich Plasma (PRP) in Rheumatoid Arthritis Patients and its Impact on Inflammatory Cytokines, Disease Activity and Quality of Life. Curr Rheumatol Rev. 2021;17(2): 232-241.
[43] VAN BUUL GM, KOEVOET WL, KOPS N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362-2370.
[44] ZHOU B, FENG H, LEI B, et al. Effect of autologous platelet-rich plasma combined with sodium hyaluronate on clinical efficacy and serum inflammatory factors in patients with knee osteoarthritis. Am J Transl Res. 2022;14(12):8724-8732.
[45] MUSUMECI G, CASTROGIOVANNI P, TROVATO FM, et al. Biomarkers of Chondrocyte Apoptosis and Autophagy in Osteoarthritis. Int J Mol Sci. 2015;16(9):20560-20575.
[46] TAO SC, YUAN T, RUI BY, et al. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7(3): 733-750.
[47] WANG Y, LUAN S, YUAN Z, et al. Therapeutic effect of platelet-rich plasma on glucocorticoid-induced rat bone marrow mesenchymal stem cells in vitro. BMC Musculoskelet Disord. 2022;23(1):151.
[48] TOMAZ DA SILVA P, ZHANG Y, Theodorakis E, et al. Cellular energy regulates mRNA degradation in a codon-specific manner. Mol Syst Biol. 2024;20(5):506-520.
[49] PINTO AP, DA ROCHA AL, MARAFON BB, et al. Impact of Different Physical Exercises on the Expression of Autophagy Markers in Mice. Int J Mol Sci. 2021;22(5):2635.
[50] XUE JF, SHI ZM, ZOU J, et al. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252-1261.
[51] XIAO L, LIN S, XU W, et al. Downregulation of Sox8 mediates monosodium urate crystal-induced autophagic impairment of cartilage in gout arthritis. Cell Death Discov. 2023;9(1):95.
[52] JIAO Y, ZHANG Q, ZHANG J, et al. Platelet-rich plasma ameliorates lipopolysaccharide-induced cardiac injury by inflammation and ferroptosis regulation. Front Pharmacol. 2022;13:1026641.
[53] CAO Y, LI Y, FU SC, et al. Platelet-rich plasma pretreatment protects anterior cruciate ligament fibroblasts correlated with PI3K-Akt-mTOR pathway under hypoxia condition. J Orthop Translat. 2022;34: 102-112.
[54] WU Z, LIANG Y, KHAN A, et al. Is occupational noise associated with arthritis? Cross-sectional evidence from US population. BMC Public Health. 2024;24(1):371.
[55] CHEN S, WU X, LAI Y, et al. Kindlin-2 inhibits Nlrp3 inflammasome activation in nucleus pulposus to maintain homeostasis of the intervertebral disc. Bone Res. 2022;10(1):5.
[56] LIU F, ZHAO Y, PEI Y, et al. Role of the NF-kB signalling pathway in heterotopic ossification: biological and therapeutic significance. Cell Commun Signal. 2024;22(1):159.
[57] QI Y, TANG R, SHI Z, et al. Wnt5a/Platelet-rich plasma synergistically inhibits IL-1β-induced inflammatory activity through NF-κB signaling pathway and prevents cartilage damage and promotes meniscus regeneration. J Tissue Eng Regen Med. 2021;15(7):612-624.
[58] INTERNATIONAL BR. Retracted: PRP from Personal Blood Relieves Joint Fluid-Inducing Synovial Injury through NF-κB Pathway and Mitochondrial Apoptosis in Human Synovial Fibroblast Cells. Biomed Res Int. 2023;2023:9875202.
[59] ZHAO H, ZHU W, MAO W, et al. Platelet-rich plasma inhibits Adriamycin-induced inflammation via blocking the NF-κB pathway in articular chondrocytes. Mol Med. 2021;27(1):66.
[60] LI Y, ZHAO B, PENG J, et al. Inhibition of NF-κB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist Updat. 2024;73:101042.
[61] VERZELLA D, CORNICE J, ARBORETTO P, et al. The NF-κB Pharmacopeia: Novel Strategies to Subdue an Intractable Target. Biomedicines. 2022; 10(9):2233.
[62] SIVAMARUTHI BS, RAGHANI N, CHORAWALA M, et al. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines. 2023;11(9):2587.
[63] CHENG Y, ZHAO Y, ZHENG Y. Therapeutic potential of triptolide in autoimmune diseases and strategies to reduce its toxicity. Chin Med. 2021;16(1):114.
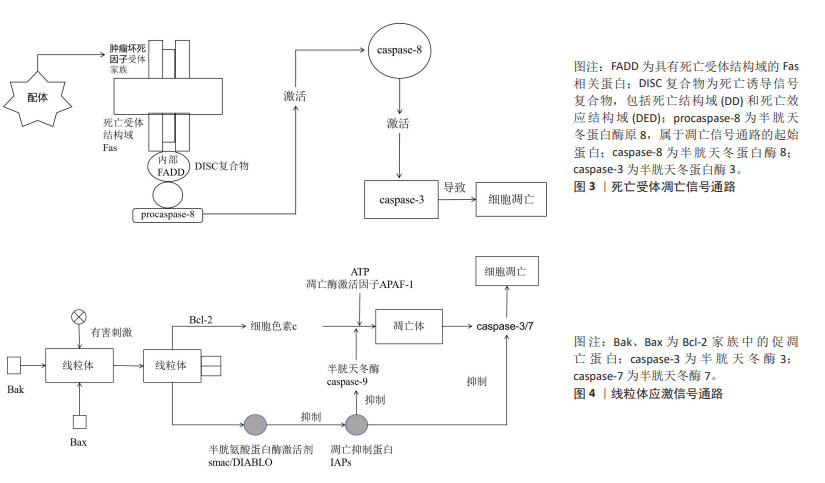
[64] OH YT, SUN SY. Regulation of Cancer Metastasis by TRAIL/Death Receptor Signaling. Biomolecules. 2021;11(4):499.
[65] XU Z, YIN W, ZHANG Y, et al. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci Rep. 2017;7:43301.
[66] SPIERINGS D, MCSTAY G, SALEH M, et al. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005; 310(5745):66-67. |