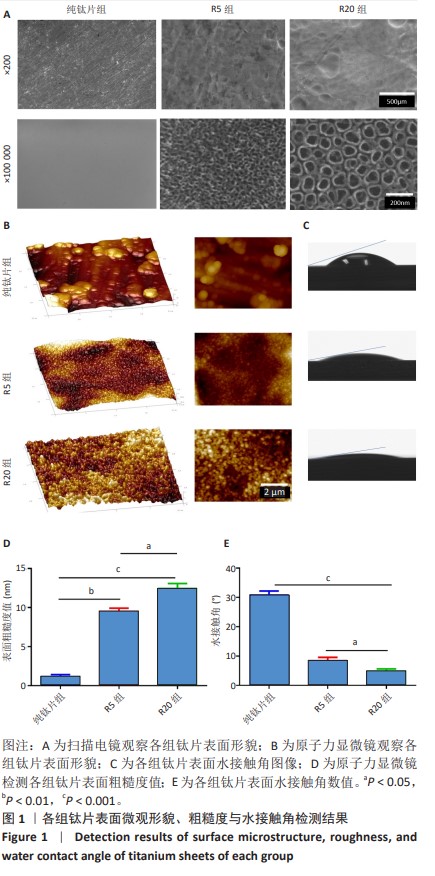
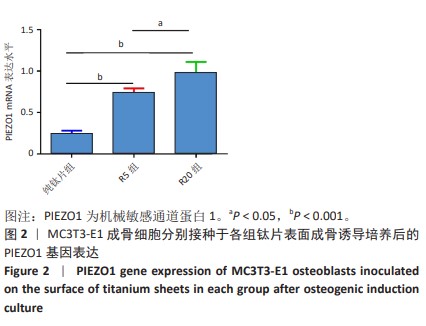
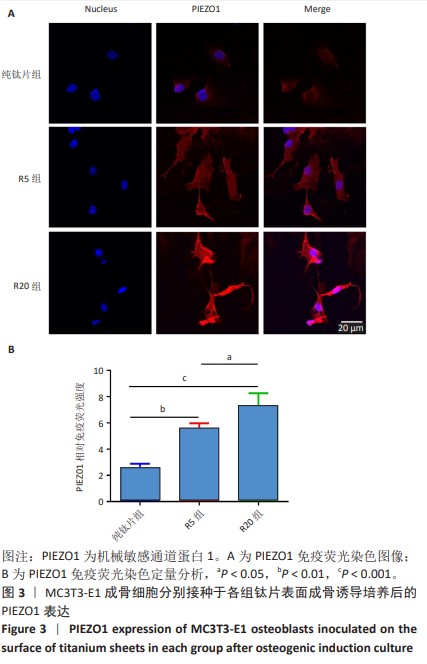
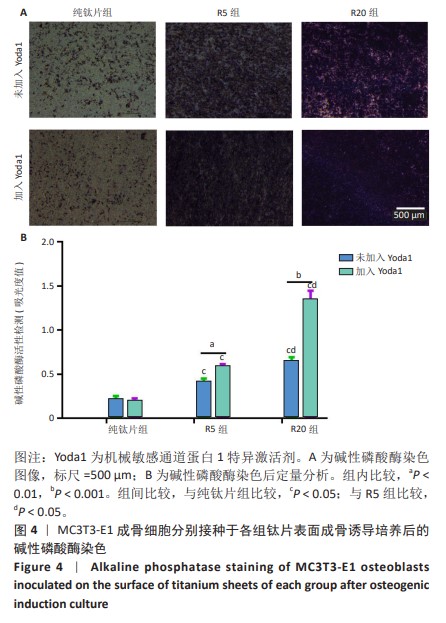
[1] 车振家,朱正清,朱礼伟,等.钛植入物表面生物化学改性对骨整合的影响[J].中国组织工程研究,2022,26(16):2576-2583.
[2] JIANG X, ZHU Y, LIU Z, et al. Association between diabetes and dental implant complications: a systematic review and meta-analysis. Acta Odontol Scand. 2021;79(1):9-18.
[3] 王旻,姜楠,祝颂松.新型钛表面微纳米共存梯度仿生结构对骨髓间充质细胞黏附、增殖及成骨分化的影响[J].口腔疾病防治, 2021,29(4):226-233.
[4] FRIBERG B, EKESTUBBE A, MELLSTRÖM D, et al. Brånemark implants and osteoporosis: a clinical exploratory study. Clin Implant Dent Relat Res. 2001;3(1):50-56.
[5] ROCCUZZO A, IMBER JC, MARRUGANTI C, et al. Clinical outcomes of dental implants in patients with and without history of periodontitis: A 20-year prospective study. J Clin Periodontol. 2022;49(12):1346-1356.
[6] PINTO TS, MARTINS BR, FERREIRA MR, et al. Nanohydroxyapatite-Blasted Bioactive Surface Drives Shear-Stressed Endothelial Cell Growth and Angiogenesis. Biomed Res Int. 2022;2022:1433221.
[7] ZHAO X, YOU L, WANG T, et al. Enhanced Osseointegration of Titanium Implants by Surface Modification with Silicon-doped Titania Nanotubes. Int J Nanomedicine. 2020;15:8583-8594.
[8] WANG W, ZHAO L, MA Q, et al. The role of the Wnt/β-catenin pathway in the effect of implant topography on MG63 differentiation. Biomaterials. 2012;33(32):7993-8002.
[9] NECULA MG, MAZARE A, ION RN, et al. Lateral Spacing of TiO(2) Nanotubes Modulates Osteoblast Behavior. Materials (Basel). 2019;12(18):2956.
[10] ZHANG Y, WANG J, HOSSEINIJENAB S, et al. Synergistic effect of nanostructure and calcium ions on improving the bioactivity of titanium implants. R Soc Open Sci. 2022;9(8):220206.
[11] WANG L, XU C, MENG K, et al. Biomimetic Hydroxyapatite Composite Coatings with a Variable Morphology Mediated by Silk Fibroin and Its Derived Peptides Enhance the Bioactivity on Titanium. ACS Biomater Sci Eng. 2023;9(1):165-181.
[12] MINAGAR S, WANG J, BERNDT CC, et al. Cell response of anodized nanotubes on titanium and titanium alloys. J Biomed Mater Res A. 2013;101(9):2726-2739.
[13] VON WILMOWSKY C, BAUER S, LUTZ R, et al. In vivo evaluation of anodic TiO2 nanotubes: an experimental study in the pig. J Biomed Mater Res B Appl Biomater. 2009;89(1):165-1671.
[14] PARK J, BAUER S, VON DER MARK K, et al. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7(6):1686-1691.
[15] BRAMMER KS, OH S, COBB CJ, et al. Improved bone-forming functionality on diameter-controlled TiO(2) nanotube surface. Acta Biomater. 2009;5(8):3215-3223.
[16] VOGEL V, SHEETZ M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7(4):265-275.
[17] DUFORT CC, PASZEK MJ, WEAVER VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12(5): 308-319.
[18] WANG J, SUN YX, LI J. The role of mechanosensor Piezo1 in bone homeostasis and mechanobiology. Dev Biol. 2023;493:80-88.
[19] LOPES HB, FREITAS GP, FANTACINI DMC, et al. Titanium with nanotopography induces osteoblast differentiation through regulation of integrin αV. J Cell Biochem. 2019;120(10):16723-16732.
[20] TSAI SW, HSU FY, CHEN PL. Beads of collagen-nanohydroxyapatite composites prepared by a biomimetic process and the effects of their surface texture on cellular behavior in MG63 osteoblast-like cells. Acta Biomater. 2008;4(5):1332-1341.
[21] DALBY MJ, GADEGAARD N, TARE R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997-1003.
[22] CHEN X, FAN H, DENG X, et al. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials (Basel). 2018;8(11):960.
[23] ZHAO L, LIU L, WU Z, et al. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials. 2012;33(9):2629-2641.
[24] ZHANG K, WANG L, LIU Z, et al. Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis. Channels (Austin). 2021;15(1):339-359.
[25] ZHANG K, LIU X, WANG L, et al. The mechanosensory and mechanotransductive processes mediated by ion channels and the impact on bone metabolism: A systematic review. Arch Biochem Biophys. 2021;711:109020.
[26] DALTON CJ, LEMMON CA. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells. 2021;10(9):2443.
[27] SZCZOT M, NICKOLLS AR, LAM RM, et al. The Form and Function of PIEZO2. Annu Rev Biochem. 2021;90:507-534.
[28] NORDGAARD C, VIND AC, STONADGE A, et al. ZAKβ is activated by cellular compression and mediates contraction-induced MAP kinase signaling in skeletal muscle. Embo J. 2022;41(17):e111650.
[29] SUAREZ RODRIGUEZ F, SANLIDAG S, SAHLGREN C. Mechanical regulation of the Notch signaling pathway. Curr Opin Cell Biol. 2023; 85:102244.
[30] SYEDA R, FLORENDO MN, COX CD, et al. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016;17(7):1739-1746.
[31] WANG Y, XIAO B. The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction. J Physiol. 2018;596(6):969-978.
[32] LIU Y, TIAN H, HU Y, et al. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int J Biol Sci. 2022; 18(10):3961-3980.
[33] SUGIMOTO A, IWATA K, KUROGOUSHI R, et al. C-terminus of PIEZO1 governs Ca2+ influx and intracellular ERK1/2 signaling pathway in mechanotransduction. Biochem Biophys Res Commun. 2023;682: 39-45.
[34] MIYAZAKI A, SUGIMOTO A, YOSHIZAKI K, et al. Coordination of WNT signaling and ciliogenesis during odontogenesis by piezo type mechanosensitive ion channel component 1. Sci Rep. 2019;9(1):14762.
[35] ZHANG G, LI X, WU L, et al. Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells. Bone Res. 2021;9(1):16.
[36] WANG L, YOU X, LOTINUN S, et al. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. 2020;11(1):282.
[37] ZHOU T, GAO B, FAN Y, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife. 2020;9:e52779. doi: 10.7554/eLife.52779.
[38] XING Y, YANG B, HE Y, et al. Effects of mechanosensitive ion channel Piezo1 on proliferation and osteogenic differentiation of human dental follicle cells. Ann Anat. 2022;239:151847.
[39] NAKASHIMA K, ZHOU X, KUNKEL G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17-29.
[40] MATSUNAGA M, KIMURA M, OUCHI T, et al. Mechanical Stimulation-Induced Calcium Signaling by Piezo1 Channel Activation in Human Odontoblast Reduces Dentin Mineralization. Front Physiol. 2021;12: 704518.
[41] SHEN B, TASDOGAN A, UBELLACKER JM, et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591(7850):438-444. |