中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (4): 846-855.doi: 10.12307/2025.214
• 生物材料综述 biomaterial review • 上一篇 下一篇
梯度人工骨修复支架调控骨骼系统组织的修复与再生
张 煜,徐睿安,方 蕾,历龙飞,刘姝妍,丁凌雪,王悦熹,郭子琰,田 丰,薛佳佳
- 有机无机复合材料国家重点实验室,生物医用材料北京实验室,先进弹性体材料研究中心,材料科学与工程学院,北京化工大学,北京市 100029
-
收稿日期:2023-10-30接受日期:2023-12-13出版日期:2025-02-08发布日期:2024-06-04 -
通讯作者:薛佳佳,博士,教授,有机无机复合材料国家重点实验室,生物医用材料北京实验室,先进弹性体材料研究中心,材料科学与工程学院,北京化工大学,北京市 100029 -
作者简介:张煜,男,2002年生,新疆维吾尔自治区人,汉族,北京化工大学本科在读,主要从事从事梯度支架在肩袖修复方面的研究。 -
基金资助:有机无机复合材料国家重点实验室开放课题(oic-20221004),项目负责人:薛佳佳
Gradient artificial bone repair scaffold regulates skeletal system tissue repair and regeneration
Zhang Yu, Xu Ruian, Fang Lei, Li Longfei, Liu Shuyan, Ding Lingxue, Wang Yuexi, Guo Ziyan, Tian Feng, Xue Jiajia
- State Key Laboratory of Organic Inorganic Composites, Beijing Laboratory of Biomedical Materials, Advanced Elastomer Materials Research Center, School of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2023-10-30Accepted:2023-12-13Online:2025-02-08Published:2024-06-04 -
Contact:Xue Jiajia, MD, Professor, State Key Laboratory of Organic Inorganic Composites, Beijing Laboratory of Biomedical Materials, Advanced Elastomer Materials Research Center, School of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China -
About author:Zhang Yu, State Key Laboratory of Organic Inorganic Composites, Beijing Laboratory of Biomedical Materials, Advanced Elastomer Materials Research Center, School of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China -
Supported by:Open Project of State Key Laboratory of Organic and Inorganic Composites, No. oic-20221004 (to XJJ)
摘要:
文题释义:
梯度支架:组成或结构特征参数在特定方向上具有一定变化的支架。骨骼系统:由骨骼、软骨、韧带和部分肌腱组成,它的主要功能是支撑身体,为人体各器官的系统运作提供保护。
背景:梯度人工骨修复支架模拟了骨骼系统中的独特特征,在骨骼系统再生中具有巨大的应用潜力。
目的:综述梯度人工骨修复支架在骨骼系统组织工程中的最新研究进展,并阐述了其优势与制造策略。
方法:由第一作者检索Web of Science和PubMed数据库2000-2023年发表的文献,英文检索词为“gradient,bone regeneration,scaffold”,最终筛选后对76篇文献进行分析总结。
结果与结论:①作为骨骼系统组织高效、高质量修复的重要手段,梯度人工骨修复支架目前针对骨组织、骨-软骨、肌腱-骨组织的天然梯度特征进行了仿生设计,这些支架能够一定程度地从结构、成分上模拟原生组织的细胞外基质,从而促进细胞黏附、迁移、增殖和分化,促进受损组织向原生状态再生恢复。②先进制造技术为梯度人工骨修复支架制备提供了更多可能;目前已经开发了通过空间差异化纤维排布和生物活性物质加载构建的梯度电纺纤维支架;分层叠加、分级孔隙率与生物3D打印技术制造的梯度3D打印支架;原位分层注射、简单逐层叠加、冷冻干燥法制造的梯度水凝胶支架;另外还包括其他方式或多方法联用的支架;这些支架在体外实验中展示了良好的生物相容性,在小型动物实验中能够加速组织再生并且观察到组织学结构明显改善。③目前开发的梯度人工骨修复支架仍需进一步优化,提高在梯度尺度上的匹配性,进一步明确材料与组织相互作用,避免降解产物导致的副反应等问题,未来需要结合相关学科优势与临床需求进一步优化。
https://orcid.org/0000-0002-7209-7935(薛佳佳)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
张 煜, 徐睿安, 方 蕾, 历龙飞, 刘姝妍, 丁凌雪, 王悦熹, 郭子琰, 田 丰, 薛佳佳. 梯度人工骨修复支架调控骨骼系统组织的修复与再生[J]. 中国组织工程研究, 2025, 29(4): 846-855.
Zhang Yu, Xu Ruian, Fang Lei, Li Longfei, Liu Shuyan, Ding Lingxue, Wang Yuexi, Guo Ziyan, Tian Feng, Xue Jiajia. Gradient artificial bone repair scaffold regulates skeletal system tissue repair and regeneration[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 846-855.
2.1.1 骨组织修复调控 骨是一种坚硬致密的组织,天然人体长骨结构由承受主要载荷的皮质骨、缓冲载荷的松质骨和输送营养的骨髓腔组成[2]。在微观尺度上,天然骨骼具有功能梯度结构,具有不同的孔径和孔隙率分布。外部致密皮质骨层的孔隙率通常为5%-10%,而内部松质骨则是小梁结构,其孔隙率为50%-90%[25],这样的结构有利于营养物质传输,从而使各种血管、神经等嵌套到有机系统中[26]。
针对天然骨组织结构的这些特点,研究人员正在尝试开发具有不同尺寸孔隙相互连接的支架,支架的空隙从几微米(促进细胞黏附)到100-500 μm(增强骨长入和毛细血管形成)并呈梯度变化[27],这些精细构建的结构梯度和进一步的仿生设计是调控骨组织修复的关键。ZHANG等[24]制备了组织良好梯度结构的3D打印硬支架模仿皮质和松质骨组织,并在此基础上注入软水凝胶,进一步赋予了支架细胞外基质的特性。
SAHAFNEJAD-MOHAMMADI等[25]在利用3D打印技术设计皮质骨-松质骨梯度支架时加入了对哈弗斯系统(一种由同心的钙化基质环组成且与表面平行,并沿骨的长轴运行的血细胞、淋巴管和神经的通道)的仿生设计,以期更大程度地接近原生骨组织结构。BOYS等[28]采用“自下而上”的策略,通过使用螯合溶液在空间上控制去除小梁骨中的矿物质来生成界面支架,控制了矿物界面化的程度和位置;这种方法制备的支架在包含结构和成分梯度以及完整的纤维状胶原结构的同时保持了组织层次结构。另外,考虑到骨组织的机械强度需要,一些研究人员通过结构梯度的设计来优化支架力学性能。例如,BARBERI等[27]利用机器人铸造技术制造了具有梯度孔隙率的生物活性玻璃支架,以便在承重部位也可用作骨替代材料;该方法可以很好地控制结构特征和尺寸,在匹配小梁骨孔的大小的基础上具有相当大的机械强度和良好的构筑性能。WU等[29]考虑到颌面骨修复支架在咬合时的应力集中问题,实现了颌面假体与种植体的一体化仿真,通过准静态加载模拟研究颌面部修复体结构优化对整个模型应力分布的影响;根据应力分布,引入晶格密度分区优化梯度结构颌面修复体。
2.1.2 骨-软骨组织修复调控 关节软骨是一种各向异性组织,其梯度分层结构在组织功能中起着关键作用。骨-软骨组织由软骨、钙化软骨和软骨下骨3部分组成,该结构决定了骨-软骨区域组织内和组织间功能的不同和梯度的存在[30]。而软骨又分为4个区域:浅层、中层、深层和钙化软骨,不同区域软骨细胞和细胞外基质有着不同的排列和组织,导致存在机械性能梯度,软骨的压缩模量和抗压强度由浅层到深层逐渐增大[31-32]。骨软骨组织存在组分梯度,比如胶原纤维类型和羟基磷灰石含量等。骨软骨组织中还具有结构梯度,包括孔隙度、孔隙大小和孔隙连通性,凝胶状的软骨孔隙率在60%-85%,而软骨下骨皮质骨孔隙率仅在5%-30%。骨软骨区域的梯度差异还体现在细胞种类与细胞数量上,软骨仅由软骨细胞组成,而骨组织由成骨细胞、破骨细胞、骨细胞、软骨细胞、内皮细胞和间充质干细胞等组成[31,33]。设计梯度人工骨修复支架,以尽可能地模仿骨和软骨的解剖、生物和物理化学特性对于促进骨-软骨再生具有重要意义。
通过设计具有上述梯度特征的支架能够影响细胞行为从而实现组织再生调控。以往的研究证明了孔径及孔几何形貌对于干细胞迁移、增殖及分化具有调节作用。CAO等[34]构建了具有孔隙率梯度且载有骨髓间充质干细胞的聚己内酯/甲基丙烯海藻酸盐复合骨软骨修复支架,该支架为3层结构,孔径变化为300 μm-500 μm-700 μm,实验证明该支架能够提高干细胞活力并促进软骨分化,具有良好的修复效果。目前同样有研究关注构建化学组分梯度支架,利用组分梯度制造梯度机械环境或直接利用化学刺激改善骨-软骨再生。PARISI等[35]构建含有低结晶羟基磷灰石颗粒梯度分布的胶原基质支架,组成和刚度的梯度优先增加了支架不同亚区域的细胞增殖,增强了不同成骨及成软骨化的能力。LIU等[36]则通过分级制造的方式构建抗炎/再生双功能梯度支架,考虑不同部位组织再生需要,在不同层级中负载相应的药物、细胞、生长因子,分区域地精准修复。另外,一些研究者重点考虑了骨软骨组织中的细胞梯度,加入生长因子控制干细胞分化。DENG等[37]构建基于丝状纤维蛋白双修饰生物支架时,在软骨层支架上负载了调控软骨细胞表型的生长因子。WEI等[38]制备的双层骨软骨水凝胶支架则进一步综合各类调控方法,引入成分刺激、生化刺激和不同孔径的梯度结构,为不同层的骨髓间充质干细胞的软骨增殖、分化提供组织特异性微环境。
2.1.3 肌腱-骨组织修复调控 肌腱-骨组织是骨骼系统中的重要组成,该组织存在的典型区域包括肩袖、髌骨肌腱和跟腱等。肌腱-骨组织界面分为4个区域,包括肌腱、未矿化的纤维软骨、矿化纤维软骨和骨骼组成,肌肉骨骼界面组织由复杂的结构梯度、成分梯度、机械性能梯度、细胞表型和对细胞功能十分重要的生化信号组成[39],其中沿界面的细胞表型梯度是渐进和连续的,不同区域之间没有明确的边界[40]。然而,这些宏观的组织学结构和复杂的梯度在损伤修复后无法重建,导致再生组织生物力学性能下降。以肩袖为例,肩袖通过肌腱-骨组织将肩胛骨周围的肌肉与肱骨相连,在发生撕裂后需要手术将断裂的肌腱锚固在肱骨上,然而在锚固处新生的血管瘢痕组织不具备肩袖原有的生物力学功能,在恢复后常二次断裂。在锚固处垫入梯度人工骨修复支架来调控再生是重建肌腱-骨组织的重要思路。
梯度人工骨修复支架在成分、结构、机械性能和细胞表型方面以分层或连续方式逐渐变化来模拟天然肌腱附着点,以促进愈合和功能恢复。一些研究聚焦于支架上的细胞负载与分化调控。例如,LYU等[41]在微流控芯片上实现了用树状流动模式来构建浓度梯度,引导干细胞分化并重塑细胞外基质,从而在脱细胞肌腱支架中培养骨髓干细胞和肌腱干细胞,实现更好的肌腱到骨组成转变。ZHU等[42]用逐层涂层的方法形成梯度,解决了细胞负载能力低和分布不均匀的问题,改善界面或矿物梯度的长度尺度匹配度,有望修复肌腱到骨的附着点。另一些则考虑模拟天然肌腱-骨组织中的结构变化,其中,胶原纤维的排列方式在逐步变化,由肌腱区域的平行排列逐渐过渡为骨区的无序排列。例如,YU等[43]用光热焊接技术实现支架的结构从有序排列到无序排列,并通过控制不同区域支架在模拟体液中的浸泡时间形成矿化梯度,这样双梯度复合设计的支架能够提高骨肌腱的再生和修复效果。CAI等[44]应用双层随机排列的纳米纤维支架形成梯度,该方法不仅可以改善骨组织的形成,还可以进一步促进胶原纤维的排列。先前的研究已经证明了天然肌腱-骨组织中存在明显的矿物梯度,在支架中重构矿物梯度是有效的修复策略。SU等[45]用流延技术将可生物降解的聚己内酯与不同含量的磷酸硅酸钙相结合层层沉积形成矿物梯度,并证明了其对细胞的定向诱导能力,伴随矿物梯度产生的刚度梯度也利于消除由肌腱收缩引起的骨肌腱连接部位的应力。YEA等[46]用钙-磷酸浸渍法形成梯度,发现脐带衍生间充质干细胞在矿物梯度支架上合成了与矿物密度相关的特定基质,如胶原蛋白、糖氨基聚糖和钙;并且在大鼠肩袖撕裂修复模型中观察到胶原组织及软骨形成量大幅度增加,再生组织的失效负荷也提高了30%,从组织学和生物力学上证明了梯度人工骨修复支架的有效性。

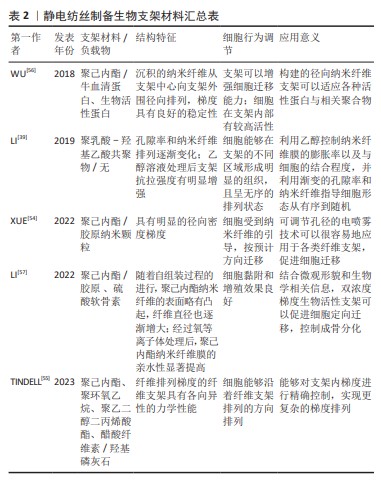
2.2.1 静电纺丝梯度支架 静电纺丝作为一种先进增材制造技术,依靠表面电荷之间的静电排斥力从黏弹性流体中射出纳米纤维[51]。因其能够加工各种材料(有机、无机及复合材料等)、调整各种性能(直径、孔隙率及厚度等)和设计各种结构(对齐、空心及芯鞘等)而被广泛研究[52]。电纺人工梯度骨修复支架的梯度可以分为2类,即结构梯度和表面梯度。结构梯度是指静电纺丝时可以通过调整聚合物溶液和静电纺丝工艺的参数实现原始纳米纤维的特性(例如排列、尺寸、成分和模量)和排列、堆叠方式,以重现各种类型组织中细胞外基质具有梯度的结构组织[53]。表面梯度是指纳米纤维的表面通过后处理用生物活性物质(蛋白、因子或矿物等)进行梯度构建,利用带有梯度的地形引导和化学线索协同操纵干细胞的迁移与分化,从而促进骨骼系统组织再生[54]。
近年来,已经有很多新方法能够实现静电纺丝结构梯度的构建。LI等[39]将聚乳酸-羟基乙酸纳米纤维膜切割成尺寸为0.5 cm×1.5 cm的矩形安装在载玻片上,放入充满乙醇蒸汽的小瓶中,以0.6 mL/min的速率滴加体积分数70%乙醇溶液,从而实现溶液掩蔽和蒸汽诱导,制备出纤维膜顶部、中部和底部区域的平均孔径分别为0.7 μm,1.3 μm和3.0 μm的梯度聚乳酸-羟基乙酸纳米纤维分级支架。CHEN等[5]提出了将二维电纺纳米纤维膜转换为具有结构和成分梯度的三维分层组合的新方法,通过减少每个连续层中加入到纳米纤维中的Pluronic F-127(聚氧乙烯聚氧丙烯醚)的含量,二维纳米纤维膜可以在气体发泡膨胀后转化为具有孔径梯度的三维组合体。用磁极的磁性辅助静电纺丝也是构建梯度的一种方法。TINDELL等[55]使用一种磁辅助静电纺丝在空间上控制纤维排列的模块化方法,静电纺丝纤维在存在磁场的情况下高度排列,并在远离磁场的情况下平稳过渡为随机排列,该方法能够沿着纤维支架的长度以亚毫米分辨率对纤维排列进行空间控制,类似于许多界面组织中存在的天然结构梯度,具有很高的发掘潜力。
静电纺丝表面梯度的研究重点则在于蛋白质梯度的构建。在再生医学中,蛋白质梯度已成为指导和影响细胞的迁移、延伸和分化以增强组织再生的有力手段,将连续蛋白质与由排列整齐的聚合物纤维制成的支架相结合可以通过加速细胞增殖和迁移来显著改善组织再生的结果[53]。通过控制沉积时间分级沉积生物活性大分子是实现构建具有连续蛋白质梯度的支架的重要策略。TANES等[53]通过线性增加引入容器惰性牛血清蛋白溶液的体积,将垂直定向的纳米纤维条竖立在容器中,在纳米纤维条的整个长度上产生惰性牛血清蛋白梯度,再用生物活性蛋白填充惰性牛血清蛋白梯度留下的空位以产生逆向梯度。考虑到很多损伤的修复需要从外周向中心招募干细胞,WU等[56]将这种技术拓展到了由径向排列的纳米纤维组成的支架上,生成了活性蛋白质的径向梯度。LI等[57]则在此基础上结合层-层自组装法开发了一种在静电纺纤维支架上生成两种生物活性成分的双梯度的通用方法,通过调整胶原蛋白和硫酸软骨素溶液在容器中的高度,将带正电荷的胶原蛋白和带负电荷的硫酸软骨素依次涂覆到单轴排列的电纺聚己内酯纳米纤维表面的特定位置。电喷雾是一种具有制造基于粒子梯度的巨大潜力的方法,这种单锥射流模式的电喷雾能够生成粒径从几百纳米到几十微米的相对均匀的聚合物颗粒。XUE等[54]在金属针和收集器之间放置一个具有可调开口尺寸的孔径,调整孔径的开口以改变纳米粒子的收集持续时间,从而导致粒子密度从收集器的中心到外围降低;并且证明了当胶原纳米粒子以径向分级方式沉积在由径向排列的纳米纤维支架上时,与均匀分布的情况相比,支架上的细胞迁移显著加速。最后,文章汇总了静电纺丝梯度支架所用材料、负载物及其他特征[39,54-57],详见表2。 通过将不同生物活性物质加入复合纳米纤维中,或是改变具体的纺丝方式,都可以搭建出具有不同机械性能和生物活性的梯度支架。除了利用静电纺丝技术进行梯度构建,研究者们还可以利用电纺丝纤维构建地形线索,调控细胞迁移,促进组织修复与癌症治疗。此外,将其他辅助技术与静电纺丝技术结合,研究者们还可以构建出性能更加优良的生物组织工程支架。然而,经典纺丝支架也存在一些问题,例如其二维膜的形态并不适应填补三维缺损,结构致密导致降解产物堆积等,纤维沉积控制难度大无法程序化设计制造等。
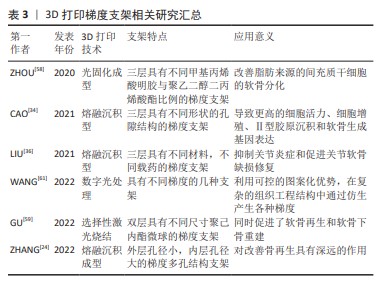
3D打印梯度支架 目前,3D打印技术借助计算机辅助制造软件可以将数字模型转化为实体,因而对骨骼系统修复时可以根据患者病情及骨骼特征等需求进行量身定制,制造出具有特定形状和结构的骨支架,在骨损伤或缺陷区域中促进新骨组织的生长。3D打印支架的梯度结构设计具体来说,是采用不同孔隙大小、形状、间距和方向的多层次结构,通过控制材料和孔隙的分布,诱导细胞分化形成硬骨、软骨及肌腱等不同类型的组织结构,以提高骨支架的机械强度、生物相容性和组织再生性。该部分将介绍用3D打印技术制备梯度支架的3种常用方式,分别为分层叠加、分级孔隙率与生物3D打印。
在进行多层叠加时,常用到各类生物可降解复合材料,其硬度的差异可以使支架对受力区域进行针对性的支撑,促进新骨的生长且可以提供不同的功能特性,如降解速率和力学特性,符合不同组织的特定需求。ZHOU等[58]使用甲基丙烯酸明胶与聚乙二醇二丙烯酸酯作为光聚合物油墨材料,分层调节两者的比例以调节支架的机械性能,并使用赖氨酸涂覆,制造出甲基丙烯酸明胶-聚乙二醇二丙烯酸酯-赖氨酸支架。具体而言,研究者首先合成3种组分比例不同的光固化油墨,将油墨程序化涂覆在平台上,当激光穿过墨水表面时,墨水发生交联并形成网格状图案;涂覆与光照依次进行直至构造出期待的三维结构,这个过程中油墨的光交联基团的种类与浓度、激光频率、强度、暴露时间都是影响支架性能的重要参数;完成打印后,经过清洗、后处理即可得到支架。GU等[59]采用上小下大的聚己内酯微球制备了双层梯度支架,由小聚己内酯微球组成的支架软骨区形成致密相,用于物理限制血管化浸润,而大聚己内酯微球制成的软骨下骨区产生多孔小梁样结构,用于促进成血管。同时,作者通过选择性激光烧结使用高能量激光束在支架上制备了不连续通道,该通道模式影响了机械强度和骨髓浸润,并触发骨软骨缺损的协同修复。
分级孔隙率的形成通常是通过软件设计和仿真工具确定孔隙率分布,将一个支架模型划分为不同的单元层或是同一层上不同的小单元,通过改变每个单元上的孔径、形状、网格比实现分级孔隙率,以适应人体形态和力学性能变化,使其更好地与周围支撑骨组织相配合。ZHANG等[24]通过熔融沉积型3D打印技术成功制备了具有梯度多孔结构的聚乳酸-透明质酸支架,模拟了天然骨组织外层的致密皮质骨与内层的多孔松质骨结构;再采用浸涂技术,将聚乳酸-透明质酸支架浸没在由甲基丙烯酸明胶分别与去铁胺/聚己内酯纳米颗粒、羰基锰纳米片、甲基丙烯酸明胶和聚丙交酯/羟基磷灰石基质混合的溶液中,以形成仿生分级支架,以增强骨修复并促进免疫系统和骨代谢的平衡。CAO等[34]打印出聚己内酯/甲基丙烯酸酯化海藻酸钠三层梯度支架。通过控制每层聚己内酯的浓度与沉积方式,制造了不同形状的孔隙结构,包括矩形、三角形和菱形,以模拟关节软骨中胶原纤维的排列,该支架具有完全相连孔和沿纵向的梯度孔,有助于细胞存活、向内生长和增殖。
生物3D打印技术通过控制生物墨水中生物材料种类、浓度,制造出不同的梯度支架,这种支架具有良好的生物相容性,其生物载荷能引起骨细胞对该结构发生重建反应,并根据不同的生物材料作用,促进血管生成、细胞增殖和分化,最终促进骨生长。LIU等[36]设计的3D生物打印多层支架,底部为聚己内酯与磷酸三钙形成的多孔支架以模拟软骨下骨小梁的结构,用于软骨下骨再生;中间层由负载kartogenin的聚己内酯和骨髓间充质干细胞负载的甲基丙烯化透明质酸生物墨水用于软骨再生的多材料交替印刷图案;顶层为双氯芬酸钠-基质金属蛋白酶-甲基丙烯酸透明质酸水凝胶层涂层,用于管理软骨损伤产生的炎症和疼痛。ZHANG等[60]制造的3D打印梯度水凝胶支架通过含有CaCl2溶液和光引发剂的海藻酸钠/聚丙烯酰胺水凝胶,以离子交联海藻酸钙作为第一网络,然后以聚丙烯酰胺作为第二网络制备可打印的生物墨水,分成3份并分别向其中混入含量占比不同的羟基磷灰石,依含量由高到低依次打印制备3层支架,该支架模拟了从软骨层到软骨下骨层所增加的刚度梯度。另外,在设计含有生物材料的梯度支架时,生物3D打印技术常与数字光处理技术一同使用。数字光处理使用数字微镜设备或液晶显示器,将设计的图案被投影到储墨器上,用于墨水光聚合,从而形成复杂的结构。这种技术可在交换梯度生物墨水时,将生物墨浪费降至最低,从而进一步节省时间和生物油墨。WANG等[61]基于数字光处理技术,利用微流体混合器生成精确可控的连续或离散梯度,制造出了多功能梯度集成的生物3D打印支架。最后,文章将3D打印梯度支架的有关信息汇总在表3中。 无论是光固化成型的3D打印技术还是选择性激光烧结与熔融沉积型的3D打印技术,都可以构建出复杂的梯度支架,而生物活性墨水的选择,极大地影响着支架的理化特征、机械性能与生物活性。3D打印技术由于能将数字模型充分转化为实体模型,其高效性与便捷性有着不可替代的优势。然而,现有3D打印支架纤维过大,表面形貌控制不佳,3D打印技术未来还需寻求更好的支架制造精度。
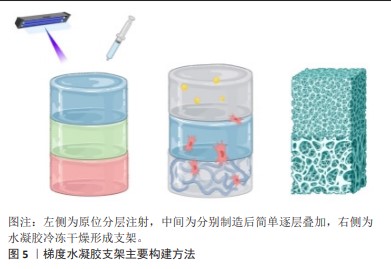
2.2.3 梯度水凝胶支架 水凝胶材料具有高储水能力、高生物相容性、低免疫原性、柔软、自然弹性特性并且降解可控,因而可以作为修复软骨和骨质缺损的材料[62]。在骨骼系统修复领域,目前有多种方式构建带有梯度的水凝胶支架,主要包括原位分层注射、简单逐层叠加和冷冻干燥法,见图5。
利用分层注射的方式,可以依次将配置好的不同水凝胶材料注射,并进行原位光交联。这种方式利用可注射水凝胶制作多层梯度水凝胶支架,并形成类似天然骨软骨组织的支架,注射可以使支架的制作和植入微创化,同时水凝胶的可塑性可以更好的填补不对称、不规则的组织缺陷。因此,可注射水凝胶是微创骨软骨组织治疗的潜在生物材料[63]。MOKHTARZADE等[62]以甲基丙烯酸明胶和琼脂糖为原料,通过改变甲基丙烯酸明胶和琼脂糖的组成比例,制备了4层支架,最上层和最下层作为软骨层,中间两层作为梯度过渡层;其中,下层软骨使用100%的明胶,向软骨层过渡过程中逐渐提高琼脂糖的含量以获得不同的支架结构;并采取逐步的方法将各层支架分别注射到组织缺损部位,并通过一系列体外测试证明了其在组织工程中的应用前途。
简单的逐层叠加技术也可以使水凝胶内部产生梯度,这种技术广泛应用于三维细胞培养研究和组织工程应用。KO等[64]通过逐层叠加技术和预交联水凝胶的方式,使水凝胶一部分交联,从而允许预水凝胶溶液与之前的水凝胶层混合,形成平滑的梯度轮廓,避免了离散层的形成,并在甲基丙烯酸明胶和聚乙二醇二丙烯酸酯水凝胶中构建牛血清白蛋白的浓度梯度,调控间充质干细胞定向迁移。CHEN等[65]模拟天然软骨和软骨下骨骼复杂的细胞外基质,通过逐层叠加技术,制造了一个异质双层水凝胶支架,其中,使用甲基丙烯酸明胶和丙烯酰葡糖胺模仿软骨细胞外基质作为上层,乙烯基膦酸纳入底层,以诱导磷酸钙的原位生物矿化;通过上层和底层水凝胶之间的相互扩散,以及添加到不同层的钙离子和海藻酸之间的螯合作用,这两个异质层被有效地融合在一起。
冷冻干燥法也是骨骼系统领域构筑梯度的常见方式,该方法通常用于模拟天然骨由致密的皮质骨到疏松的小梁骨的孔径梯度结构[66]。PITROLINO等[67]使用纳米羟基磷灰石和壳聚糖,结合冷冻干燥和孔源浸出的方法,制造了一种多孔的、生物可吸收的支架,其孔径有明显的梯度,两个支架中每个部分的孔隙大小是通过添加聚己内酯微球的筛分物来控制,再利用冷冻干燥实现孔隙互联,模仿了骨软骨组织的自然结构和化学成分。除了结合孔源浸出法,RAUCCI等[68]结合冷冻干燥和溶胶-凝胶法这两种技术,证明了制造具有特定生物活性信号分布的复合水凝胶支架的可行性,基于磷酸钙溶胶-凝胶转变的化学方法确保了颗粒分散到明胶基质中,并直接控制羧基明胶与钙离子之间的相互作用。另外,还探讨了制造方法与配方对羟基磷灰石浓度及形貌的影响。
人工合成的生物基水凝胶有着明显的稳定性,制备复合生物基水凝胶材料,还可以提升其生物活性。采用不同的水凝胶制备方法,所构建支架的性能也有一定差异。由于水凝胶材料能较好地修补不规则组织缺陷,其应用前景广,而开发新型水凝胶方法,不仅可以改善支架性能,也可以使支架的微观尺度更贴近真实生物组织支架。然而,水凝胶材料力学性能较弱、降解速率过快等因素限制了其应用,开发更接近原生组织力学性能以及匹配组织再生速率的水凝胶材料非常重要。
2.2.4 其他方法与多方法联用构建梯度支架 除了上述提及的3种主要方法外,还有其他一些构建人工梯度骨修复支架的方法。例如,BAI等[69]尝试克服羟基磷灰石加工成型困难的问题,开发了一种改进的冷冻铸造技术来制备具有梯度通道结构的陶瓷支架,设计了能够产生自发毛细管流动的梯度通道结构,利用梯度通道基部到末端的毛细流动效应促进细胞在支架上的接种。NIE等[70]开发了一种新型多层支架粘结方法,构建了通过内源性纤维化软骨细胞外基质将软骨水凝胶和烧结聚乳酸-羟基乙酸微球支架结合来制造多相移植物。另外,考虑到现有多层支架相邻层间的梯度突变不利于平滑组织界面的形成,许多研究人员正在尝试构建一体化的支架,以实现可靠的组织整合。ZHU等[71]以明胶微球在离心管中组装成立方密堆积晶格作为模板,利用羟基磷灰石纳米颗粒在重力下的沉降构建矿物梯度,然后用聚乳酸-羟基乙酸溶液固定困在空隙中的羟基磷灰石,冷冻干燥并去除明胶模板后,获得了具有矿物质含量梯度的多孔支架。QIU等[72]通过羟基磷灰石/聚己内酯薄膜在聚己内酯溶液中的溶胀,利用界面扩散在可生物降解的支架中制造控制良好的矿物梯度,得到了与人体肌腱附着点中矿物质梯度的长度尺度相当的平滑梯度人工骨修复支架。
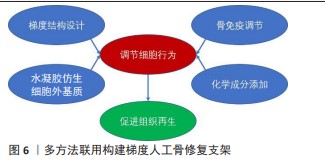
目前已经发展了多种梯度人工骨修复支架的制造方法,不同的方法可以在一定程度上联合并用,并且进一步与组织工程方法和生长因子等活性物质递送结合。例如,可以用具有梯度结构的3D打印硬支架模仿皮质和松质骨组织并在内部注入软水凝胶进一步赋予了支架细胞外基质的特性,然后在此基础上添加更多参与免疫调节的分子或其他调节细胞行为的化学物质[24],见图6。另外,水凝胶同3D打印支架结合可以构建药物缓释系统,调节间充质干细胞的行为。例如,WANG等[73]设计了具有适合软骨和骨骼形成的双层梯度孔径的3D打印聚己内酯支架,以提供结构和机械支撑,为了连续调节骨髓间充质干细胞的行为,使用快速降解的海藻酸钠水凝胶释放E7肽以增强早期骨髓间充质干细胞的迁移,同时使用缓慢降解的丝素蛋白多孔基质根据水凝胶和丝素蛋白基质的不同降解率,提供B2A肽的持续释放,使后期骨髓间充质干细胞能够向成骨细胞和软骨细胞进行双向分化。
| [1] KOUSHIK TM, MILLER CM, ANTUNES E. Bone tissue engineering scaffolds: function of multi-material hierarchically structured scaffolds. Adv Healthc Mater. 2023;12(9):2202766. [2] BAI X, GAO M, SYED S, et al. Bioactive hydrogels for bone regeneration. Bioact Mater. 2018;3(4):401-417. [3] BAAWAD A, JACHO D, HAMIL T, et al. Polysaccharide-based composite scaffolds for osteochondral and enthesis regeneration. Tissue Eng Part B Rev. 2023;29(2):123-140. [4] CHEN P, LI L, DONG L, et al. Gradient biomineralized silk fibroin nanofibrous scaffold with osteochondral inductivity for integration of tendon to bone. ACS Biomater Sci Eng. 2021;7(3):841-851. [5] CHEN S, MCCARTHY A, JOHN JV, et al. Converting 2D nanofiber membranes to 3D hierarchical assemblies with structural and compositional gradients regulates cell behavior. Adv Mater. 2020;32(43):2003754. [6] YANG R, ZHENG Y, ZHANG Y, et al. Bipolar metal flexible electrospun fibrous membrane based on metal-organic framework for gradient healing of tendon-to-bone interface regeneration. Adv Healthc Mater. 2022;11(12):2200072. [7] YILDIRIM N, AMANZHANOVA A, KULZHANOVA G, et al. Osteochondral interface: regenerative engineering and challenges. ACS Biomater Sci Eng. 2023;9(3):1205-1223. [8] LI C, OUYANG L, ARMSTRONG JPK, et al. Advances in the fabrication of biomaterials for gradient tissue engineering. Trends Biotechnol. 2021;39(2): 150-164. [9] ZHU C, QIU J, THOMOPOULOS S, et al. Augmenting tendon-to-bone repair with functionally graded scaffolds. Adv Healthc Mater. 2021;10(9):e2002269. [10] XUE J, WU T, QIU J, et al. Promoting cell migration and neurite extension along uniaxially aligned nanofibers with biomacromolecular particles in a density gradient. Adv Funct Mater. 2020;30(40):2002031. [11] SHERWOOD JK, RILEY SL, PALAZZOLO R, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23(24):4739-4751. [12] FAIA-TORRES AB, GUIMOND-LISCHER S, ROTTMAR M, et al. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014;35(33):9023-9032. [13] LEE CH, RODEO SA, FORTIER LA, et al. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med. 2014;6(266):266ra171. [14] LI X, XIE J, LIPNER J, et al. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009; 9(7):2763-2768. [15] LIU W, LIPNER J, XIE J, et al. Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl Mater Interfaces. 2014; 6(4):2842-2849. [16] PHILLIPS JE, BURNS KL, LE DOUX JM, et al. Engineering graded tissue interfaces. Proc Natl Acad Sci U S A. 2008;105(34):12170-12175. [17] WOODFIELD TBF, VAN BLITTERSWIJK CA, DE WIJN J, et al. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng. 2005;11(9-10):1297-1311. [18] DORMER NH, SINGH M, WANG L, et al. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng. 2010;38(6):2167-2182. [19] KON E, DELCOGLIANO M, FILARDO G, et al. Novel nano-composite multilayered biomaterial for osteochondral regeneration a pilot clinical trial. Am J Sports Med. 2011;39(6):1180-1190. [20] NGUYEN LH, KUDVA AK, SAXENA NS, et al. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32(29):6946-6952. [21] SUBRAMONY SD, DARGIS BR, CASTILLO M, et al. The guidance of stem cell differentiation by substrate alignment and mechanical stimulation. Biomaterials. 2013;34(8):1942-1953. [22] GAO F, XU Z, LIANG Q, et al. Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv Sci. 2019;6(15):1900867. [23] GAO F, XU Z, LIANG Q, et al. Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv Funct Mater. 2018;28(13):1706644. [24] ZHANG J, TONG D, SONG H, et al. Osteoimmunity-regulating biomimetically hierarchical scaffold for augmented bone regeneration. Adv Mater. 2022; 34(36):e2202044. [25] SAHAFNEJAD-MOHAMMADI I, RAHMATI S, NAJMODDIN N, et al. Biomimetic polycaprolactone-graphene oxide composites for 3d printing bone scaffolds. Macromol Mater Eng. 2023;308(5):2200558. [26] LIAO B, XIA RF, LI W, et al. 3D-printed Ti6Al4V scaffolds with graded triply periodic minimal surface structure for bone tissue engineering. J Mater Eng Perform. 2021;30(7):4993-5004. [27] BARBERI J, BAINO F, FIUME E, et al. Robocasting of SiO2-based bioactive glass scaffolds with porosity gradient for bone regeneration and potential load-bearing applications. Materials. 2019;12(17):2691. [28] BOYS AJ, ZHOU H, HARROD JB, et al. Top-down fabrication of spatially controlled mineral-gradient scaffolds for interfacial tissue engineering. ACS Biomater Sci Eng. 2019;5(6):2988-2997. [29] WU H, CHAO L, ZHANG Q, et al. Design and 3D printing of ceramic maxillofacial prosthesis with gradient pores based on Voronoi-Tessellation principle. Mater Today Commun. 2022;33:104559. [30] KHORSHIDI S, KARKHANEH A. A review on gradient hydrogel/fiber scaffolds for osteochondral regeneration. J Tissue Eng Regener Med. 2018;12(4): e1974-e1990. [31] ANSARI S, KHORSHIDI S, KARKHANEH A. Engineering of gradient osteochondral tissue: from nature to lab. Acta Biomater. 2019;87: 41-54. [32] DI LUCA A, VAN BLITTERSWIJK C, MORONI L. The osteochondral interface as a gradient tissue: From development to the fabrication of gradient scaffolds for regenerative medicine. Birth Defects Res C Embryo Today Rev. 2015;105(1):34-52. [33] NAGHIZADEH Z, KARKHANEH A, KHOJASTEH A. Self-crosslinking effect of chitosan and gelatin on alginate based hydrogels: injectable in situ forming scaffolds. Mater Sci Eng C. 2018;89:256-264. [34] CAO Y, CHENG P, SANG S, et al. Mesenchymal stem cells loaded on 3D-printed gradient poly(ε-caprolactone)/methacrylated alginate composite scaffolds for cartilage tissue engineering. Regener Biomater. 2021;8(3): rbab019. [35] PARISI C, SALVATORE L, VESCHINI L, et al. Biomimetic gradient scaffold of collagen–hydroxyapatite for osteochondral regeneration. J Tissue Eng. 2020;11:2041731419896068. [36] LIU Y, PENG L, LI L, et al. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279:121216. [37] DENG C, YANG J, HE H, et al. 3D bio-printed biphasic scaffolds with dual modification of silk fibroin for the integrated repair of osteochondral defects. Biomater Sci. 2021;9(14):4891-4903. [38] WEI W, LIU W, KANG H, et al. A one-stone-two-birds strategy for osteochondral regeneration based on a 3D printable biomimetic scaffold with kartogenin biochemical stimuli gradient. Adv Healthc Mater. 2023; 12(15):e2300108. [39] LI H, WU T, XUE J, et al. Transforming nanofiber mats into hierarchical scaffolds with graded changes in porosity and/or nanofiber alignment. Macromol Rapid Commun. 2019;41(3):1900579. [40] DANG GP, QIN W, WAN QQ, et al. Regulation and reconstruction of cell phenotype gradients along the tendon-bone interface. Adv Funct Mater. 2023;33(2):2210275. [41] LYU J, CHEN L, ZHANG J, et al. A microfluidics-derived growth factor gradient in a scaffold regulates stem cell activities for tendon-to-bone interface healing. Biomater Sci. 2020;8(13):3649-3663. [42] ZHU C, PONGKITWITOON S, QIU J, et al. Design and fabrication of a hierarchically structured scaffold for tendon-to-bone repair. Adv Mater. 2018;30(16):1707306. [43] YU C, WANG T, DIAO H, et al. Photothermal-triggered structural change of nanofiber scaffold integrating with graded mineralization to promote tendon–bone healing. Adv Fiber Mater. 2022;4(4):908-922. [44] CAI J, WANG J, YE K, et al. Dual-layer aligned-random nanofibrous scaffolds for improving gradient microstructure of tendon-to-bone healing in a rabbit extra-articular model. Int J Nanomed. 2018;13:3481-3492. [45] SU W, GUO J, XU J, et al. Gradient composite film with calcium phosphate silicate for improved tendon -to-Bone intergration. Chem Eng J. 2021;404:126473. [46] YEA JH, BAE TS, KIM BJ, et al. Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 2020;114:104-116. [47] ZHANG M, QIN C, WANG Y, et al. 3D printing of tree-like scaffolds for innervated bone regeneration. Addit Manuf. 2022;54:102721. [48] CHEN L, WEI L, SU X, et al. Preparation and characterization of biomimetic functional scaffold with gradient structure for osteochondral defect repair. Bioengineering-Basel. 2023;10(2):213. [49] HUA D, XIONG R, BRAECKMANS K, et al. Concentration gradients in material sciences:methods to design and biomedical applications. Adv Funct Mater. 2021;31(15):2009005. [50] DU YY, GUO JL, WANG JL, et al. Hierarchically designed bone scaffolds:From internal cues to external stimuli. Biomaterials. 2019;218:119334. [51] XUE J, WU T, XIA Y. Perspective: aligned arrays of electrospun nanofibers for directing cell migration. APL Mater. 2018;6(12):120902. [52] ZHAO T, ZHANG J, GAO X, et al. Electrospun nanofibers for bone regeneration: from biomimetic composition, structure to function. J Mater Chem B. 2022;10(32):6078-6106. [53] TANES ML, XUE J, XIA Y. A general strategy for generating gradients of bioactive proteins on electrospun nanofiber mats by masking with bovine serum albumin. J Mater Chem B. 2017;5(28):5580-5587. [54] XUE J, WU T, QIU J, et al. Accelerating cell migration along radially aligned nanofibers through the addition of electrosprayed nanoparticles in a radial density gradient. Part Part Syst Charact. 2022;39(4):2100280. [55] TINDELL RK, BUSSELLE LP, HOLLOWAY JL. Magnetic fields enable precise spatial control over electrospun fiber alignment for fabricating complex gradient materials. J Biomed Mater Res Part A. 2023;111(6):778-789. [56] WU T, XUE J, LI H, et al. General method for generating circular gradients of active proteins on nanofiber scaffolds sought for wound closure and related applications. ACS Appl Mater Interfaces. 2018;10(10):8536-8545. [57] LI L, WANG T, VAN K, et al. Dual gradients of bioactive components on electrospun fibers for cell migration and controlled stem cell differentiation. Mater Today Adv. 2022;16:100301. [58] ZHOU X, TENAGLIO S, ESWORTHY T, et al. Three-dimensional printing biologically inspired dna-based gradient scaffolds for cartilage tissue regeneration. ACS Appl Mater Interfaces. 2020;12(29):33219-33228. [59] GU X, ZHA Y, LI Y, et al. Integrated polycaprolactone microsphere-based scaffolds with biomimetic hierarchy and tunable vascularization for osteochondral repair. Acta Biomater. 2022;141:190-197. [60] ZHANG H, HUANG H, HAO G, et al. 3D printing hydrogel scaffolds with nanohydroxyapatite gradient to effectively repair osteochondral defects in rats. Adv Funct Mater. 2020;31(1):2006697. [61] WANG M, LI W, MILLE LS, et al. Digital light processing based bioprinting with composable gradients. Adv Mater. 2022;34(1):2107038. [62] MOKHTARZADE A, IMANI R, SHOKROLLAHI P. A gradient four-layered gelatin methacrylate/agarose construct as an injectable scaffold for mimicking osteochondral tissue. J Mater Sci. 2023;58(13):5735-5755. [63] LIU M, ZENG X, MA C, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5(1):17014. [64] KO H, SUTHIWANICH K, MARY H, et al. A simple layer-stacking technique to generate biomolecular and mechanical gradients in photocrosslinkable hydrogels. Biofabrication. 2019;11(2):025014. [65] CHEN Z, XIAO H, ZHANG H, et al. Heterogenous hydrogel mimicking the osteochondral ECM applied to tissue regeneration. J Mater Chem B. 2021; 9(41):8646-8658. [66] SHI D, SHEN J, ZHANG Z, et al. Preparation and properties of dopamine-modified alginate/chitosan–hydroxyapatite scaffolds with gradient structure for bone tissue engineering. J Biomed Mater Res Part A. 2019;107(8): 1615-1627. [67] PITROLINO KA, FELFEL RM, PELLIZZERI LM, et al. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr Polym. 2022;282:119126. [68] RAUCCI MG, DEMITRI C, SORIENTE A, et al. Gelatin/nano-hydroxyapatite hydrogel scaffold prepared by sol-gel technology as filler to repair bone defects. J Biomed Mater Res Part A. 2018;106(7):2007-2019. [69] BAI H, WANG D, DELATTRE B, et al. Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect. Acta Biomater. 2015;20:113-119. [70] NIE X, CHUAH YJ, HE P, et al. Engineering a multiphasic, integrated graft with a biologically developed cartilage-bone interface for osteochondral defect repair. J Mater Chem B. 2019;7(42):6515-6525. [71] ZHU C, QIU J, PONGKITWITOON S, et al. Inverse opal scaffolds with gradations in mineral content for spatial control of osteogenesis. Adv Mater. 2018;30(29):1706706. [72] QIU J, AHN J, QIN D, et al. Biomimetic scaffolds with a mineral gradient and funnel-shaped channels for spatially controllable osteogenesis. Adv Healthc Mater. 2022;11(9):2100828. [73] WANG Y, LING C, CHEN J, et al. 3D-printed composite scaffold with gradient structure and programmed biomolecule delivery to guide stem cell behavior for osteochondral regeneration. Biomater Sci. 2022;140:213967. [74] ZHANG X, LI L, OUYANG J, et al. Electroactive electrospun nanofibers for tissue engineering. Nano Today. 2021;39:101196. [75] ZHANG B, HUANG J, NARAYAN RJ. Gradient scaffolds for osteochondral tissue engineering and regeneration. J Mater Chem B. 2020;8(36):8149-8170. [76] ZELAYA-LAINEZ L, KARIEM H, NISCHKAUER W, et al. “Variances” and “in-variances” in hierarchical porosity and composition, across femoral tissues from cow, horse, ostrich, emu, pig, rabbit, and frog. Mater Sci Eng C. 2020;117:111234 |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 王自林, 牟秋菊, 刘宏杰, 申玉雪, 祝丽丽. 载富血小板血浆水凝胶对L929细胞氧化损伤的保护作用[J]. 中国组织工程研究, 2025, 29(4): 771-779. |
| [3] | 赵红霞, 孙政伟, 韩 阳, 吴学超, 韩 静. 富血小板纤维蛋白复合甲基丙烯酰化明胶水凝胶的促成骨性能[J]. 中国组织工程研究, 2025, 29(4): 809-817. |
| [4] | 孙现娟, 王秋花, 张锦艺, 杨杨杨, 王文双, 张晓晴. 不同静电纺丝膜上骨髓间充质干细胞的黏附、增殖与成血管平滑肌分化[J]. 中国组织工程研究, 2025, 29(4): 661-669. |
| [5] | 赵增波, 李晨曦, 窦晨雷, 马 娜, 周冠军. 壳聚糖/甘油磷酸钠/海藻酸钠/益母草碱水凝胶的抗炎与促成骨作用[J]. 中国组织工程研究, 2025, 29(4): 678-685. |
| [6] | 董美林, 都海宇, 刘 源. 负载槲皮素的羧甲基壳聚糖水凝胶促进糖尿病大鼠创面愈合[J]. 中国组织工程研究, 2025, 29(4): 692-699. |
| [7] | 张 博, 张 振, 江 东. 单宁酸改性互穿网络水凝胶促进断裂跟腱术后的组织重塑[J]. 中国组织工程研究, 2025, 29(4): 721-729. |
| [8] | 吴 辰, 江佳慧, 苏 豆, 刘 晨, 慈 超. 脱细胞皮肤基质/聚氨酯混纺纤维支架促进大鼠皮肤缺损的修复[J]. 中国组织工程研究, 2025, 29(4): 745-751. |
| [9] | 姜至秀, 季俣辰, 刘丹瑜, 曹怡琳, 姜婷婷, 宋颐函, 王 磊, 王心彧. Gyroid结构钛仿生骨支架修复下颌骨节段性缺损的生物力学性能[J]. 中国组织工程研究, 2025, 29(22): 4621-4628. |
| [10] | 闫星华, 王心彧, 刘 苗, 韩泽奎, 宋颐函, 张 岩, 孙子惠. 亲水性Gyroid结构种植体的制备及力学性能分析[J]. 中国组织工程研究, 2025, 29(16): 3343-3350. |
| [11] | 李永航, 李文铭, 严才平, 王星宽, 向 超, 张 袁, 蒋 科, 陈 路. 抗纤维化与促“H”型血管核壳结构生物支架修复临界骨缺损[J]. 中国组织工程研究, 2025, 29(16): 3420-3431. |
| [12] | 朱文博, 张旭婧, 许 燕, 石欣桐. 基于三周期极小曲面晶胞梯度支架的设计及力学性能[J]. 中国组织工程研究, 2025, 29(16): 3449-3457. |
| [13] | 何 蕊, 李重一, 王瑞瑶, 曾 丹, 范代娣. MXene基水凝胶在创面修复领域的应用[J]. 中国组织工程研究, 2025, 29(16): 3486-3493. |
| [14] | 刘忠钰, 李文娅, 范永鸿, 吕 双, 裴 娟, 陈娅琴, 刘倍余, 孙红玉. 甲基丙烯酰化改性真皮细胞外基质水凝胶促进腹壁缺损修复[J]. 中国组织工程研究, 2025, 29(10): 2074-2082. |
| [15] | 吴尧昆, 刘成林, 付佳豪, 宋 伟, 陈 浩, 席洪钟, 刘 锌, 杜 斌, 孙光权. 中药有效成分结合骨组织工程材料用于骨修复[J]. 中国组织工程研究, 2025, 29(10): 2141-2150. |
尽管在以往的研究中已经利用多种方法开发了各类人工骨修复支架,但常因为忽视骨骼肌组织中存在的梯度,导致治疗效果不佳。作为生物学的普遍特征,梯度在组织发育和生理学中具有重要的功能作用[8],这些梯度可以大致分为结构梯度和成分梯度。成分梯度是细胞外基质成分的转变,例如,在骨-软骨组织存在显著的羟基磷灰石含量梯度,并且对间充质干细胞行为和组织机械性能的分级转变有重要影响;而胶原蛋白、蛋白多糖和水化梯度存在于整个关节软骨中[7,9],见图1A。结构梯度是组织结构的改变,例如皮质骨中存在的孔隙度变化或肩袖组织中纤维取向度的差异,见图1B,C,这些梯度的匹配对新生组织和原生组织的生物整合具有重要作用。这些存在于各类组织中的梯度为人工骨修复支架的设计提供了仿生学灵感,通过梯度人工骨修复支架的构建,可以调控骨骼及骨界面组织的再生,从而重构特殊生理结构和复杂的机械环境。另外,细胞迁移也经常响应浓度梯度而发生,例如单向梯度的胶原蛋白颗粒能促进骨髓干细胞沿颗粒密度增加的方向迁移[8,10]。通过生物活性蛋白浓度梯度的构建,能够募集内源性干细胞到缺损部位增殖分化以促进组织再生。因此,具有梯度特征的人工骨修复支架引起了研究者的关注。例如,CHEN等[4]制备了一种具有梯度矿物涂层的纳米纤维支架并证实了纳米纤维支架不同区域具有不同的矿化程度,力学性能各不相同,对骨髓间充质干细胞生长和分化的影响也不同,实现肌腱-骨组织的整合增强,具有更高的极限负荷和更多的纤维软骨样组织形成。
以往的综述常专注于单一组织的修复并概括其组织工程的修复策略,或者材料及制造技术在组织修复领域的应用,但此综述概括了骨骼系统中各组织的梯度特征,总结了梯度人工骨修复支架的设计及制造策略,重点阐明了如何利用或模拟原生组织中存在的梯度来设计人工骨修复支架,以改善组织再生。另外,文章关注了梯度人工骨修复支架的先进制造方法,总结了近年来构筑梯度人工骨修复支架的制造策略与制备方法,为未来组织修复及人工骨修复支架的制造提供了一种新的思路及策略。
1.1.1 检索人及检索时间 第一作者在2023年4月进行检索。
1.1.2 检索文献时限 2000年1月至2023年4月。
1.1.3 检索数据库 Web of Science和PubMed数据库。
1.1.4 检索词 英文检索词为“gradient,bone regeneration,bone decfect,scaffold”。
1.1.5 检索文献类型 研究原著、综述、述评、临床试验和病例报告。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以PubMed数据库检索策略为例,见图2。
1.1.8 检索文献量 初步检索得到Web of Science数据库英文文献494篇,PubMed数据库276篇。
1.2 入组标准
1.2.1 纳入标准 ①研究内容为构建调控骨骼系统组织再生的人工支架,并必须模拟原生组织细胞外基质梯度特征;②具有创新性的较高质量研究型文章,研究内容详实完整;③总结性强、立意明确、文献来源可靠及观点阐述清晰的综述文章。
1.2.2 排除标准 ①文章内容与此综述相关性不强,以其他组织修复为目标或主要创新点与梯度无关;②发表时间过早、参考价值不高及内容类似的同类研究。
1.3 文献质量评估和数据的提取 ①文献创新性强、研究意义明确;②研究思路清晰、实验设计合理;③文章内容完整,必须包括支架制备、生物相容性评价和效果评估部分。
1.4 数据提取 Web of Science数据库共检索到494篇文献,PubMed数据库共检索到276篇文献,比较删去重复及无法获取文献后共得到文献383篇,阅读标题及摘要后排除得到123篇,对123篇文献进行大致的粗略阅读及质量评估,最终纳入76篇符合标准的文献进行综述。最终入选文献分布为Web of Science和PubMed均可查到43篇,Web of Science数据库独有文献33篇,文献筛选流程见图3。
3.2 作者综述区别于他人他篇的特点 此综述着重关注了骨骼系统中存在的梯度特征,主要包括骨骼自身、骨-软骨和肌腱-骨3种组织,并阐述了构建具有梯度的人工骨修复支架在这些组织治疗中的优势与常用策略。另外,研究者们总结了近年来梯度人工骨修复支架的先进制造方式,着重关注了静电纺丝、3D打印和水凝胶3种常用方法的研究进展,并列举了其他具有潜力的制造方法,阐述了多方法协同制造梯度人工骨修复支架的优势。
3.3 综述的局限性 ①此综述主题所在领域医学与材料科学高度交叉,受行文结构与作者研究内容影响,此综述未能全面地提供医学方面的背景知识,仅简单介绍了组织的梯度特征,用以阐述梯度人工骨修复支架的构建思路。②由于梯度人工骨修复支架修复的部位和损伤的情况差异悬殊,实验方法和动物模型也不尽相同,因此此综述未能比较出修复效果最佳的材料或制造方式。
3.4 综述的重要意义 骨骼系统组织修复与再生是根治众多骨科疾病、改善患者治疗预后的重要方式。考虑到骨骼肌组织中存在包括结构、组分、机械性能及细胞表型在内的多种梯度,具有梯度的人工骨修复支架在相关组织修复与再生领域具有很大的潜力。梯度人工骨修复支架能够模拟细胞外基质,为细胞提供地形、生化线索,促进细胞迁移、增殖和分化,从而促进组织再生。随着再生医学的进步,梯度人工骨修复支架有望进一步提高疗效,向临床及商品化推进。
3.5 课题专家组对未来的建议 ①首先,现有支架的设计总体上仿生程度较低,以宏观组织层面的仿生为主。一方面是对骨-肌腱和骨-软骨等组织的结构与成分仍需进一步的探索,并且探讨组织亚结构单元仿生的必要性与可能性;另一方面,也需要更深入探索天然组织中的特征梯度与其分布方式,并与之更匹配地设计支架中的梯度,比如现有一些分层梯度支架的突变边界不利于平滑组织界面的形成,另有一些则梯度跨越远超天然组织的长度尺度[9]。②其次,强化梯度人工骨修复支架对细胞行为的调节作用仍然是重要的发展方向,目前许多研究人员在支架提供地形线索的基础上引入了生长因子、水凝胶或免疫调节分子,但在用法用量及系统化调控等方面仍有很大的探索空间;另外,已经有相当多的研究证实了材料微纳米形貌能通过机械刺激、免疫调节、蛋白质吸附等方式调节细胞行为,未来可以在支架中引入合适的微米、纳米级的形貌设计以调节细胞的增殖、分化,并尝试定向引导细胞黏附和改善再生组织性能;值得注意的是,骨骼系统与运动及受力息息相关,如果在支架设计中引入机械刺激与生物力学方面的考量可能会有利于组织修复再生[39,74]。③最后,从体内组织愈合再生的角度考虑,一方面愈合是一个较为复杂、长期的过程,梯度人工骨修复支架可以着眼于适应不同愈合阶段的需求,利用刺激响应材料来赋能“智能”支架,精准可控地实现调节;另一方面,组织愈合再生的环境随着患者身体状况、受损部位、受损情况而复杂多变,比如根据患者缺损的大小与形状个性化定制支架就需要依托人工智能与医学影像学发展来利用高分辨率的成像捕获缺损信息辅助支架设计,并且探索实验动物与人类组织的共性与不同并建立合适的动物模型[75-76]。相信梯度人工骨修复支架在未来能够得到更好的发展,造福人类健康事业。
文题释义:
梯度支架:组成或结构特征参数在特定方向上具有一定变化的支架。骨骼系统:由骨骼、软骨、韧带和部分肌腱组成,它的主要功能是支撑身体,为人体各器官的系统运作提供保护。
文章综述了近年来梯度人工骨修复支架用于调控骨骼系统组织再生的研究进展。着重阐明了如何从模拟原生组织梯度的角度设计人工骨修复支架及梯度人工骨修复支架用于骨骼系统组织再生的优势。利用梯度人工骨修复支架提供的地形和生化线索,调控细胞迁移、增殖、分化,从而促进组织再生,并总结了利用先进制造技术制备梯度人工骨修复支架的各种主要方法,概述了各自的特征、优势与不足。讨论了其他新兴制造方式与多方法联用制造梯度人工骨修复支架的优势。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||