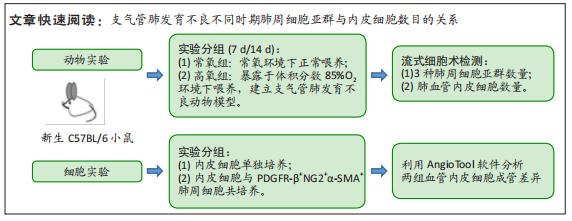
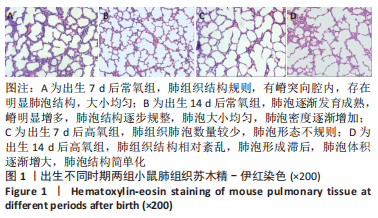
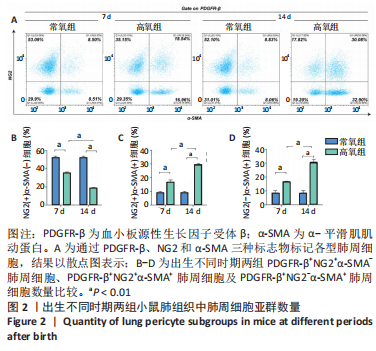
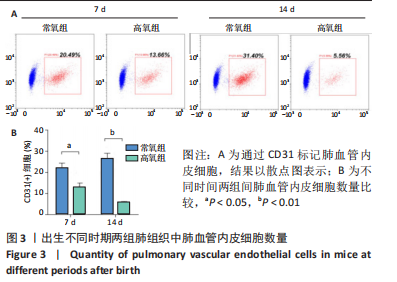
[1] JOSHI S, KOTECHA S. Lung growth and development. Early Hum Dev. 2007;(12):83.
[2] MORRISEY EE, HOGAN B. Preparing for the First Breath: Genetic and Cellular Mechanisms in Lung Development. Dev Cell. 2010;18(1):8-23.
[3] WHITE ES. Commentary: A Breath of Fresh Air on the Mesenchyme: Impact of Impaired Mesenchymal Development on the Pathogenesis of Bronchopulmonary Dysplasia. Front Med (Lausanne). 2016;3:13.
[4] LU X, GONG J, DENNERY PA, et al. Endothelial-to-mesenchymal transition: Pathogenesis and therapeutic targets for chronic pulmonary and vascular diseases. Biocheml Pharmacol. 2019;168:100-107.
[5] 翟丽丽,杨迷玲,李争艳,等.周细胞的研究进展[J].现代肿瘤医学,2011,19(8):4.
[6] Dessalles CA, Babataheri A, Barakat AI. Pericyte mechanics and mechanobiology. J Cell Sci. 2021;134(6):jcs240226.
[7] HUNG CF, WILSON CL, SCHNAPP LM. Pericytes in the Lung. Adv Exp Med Biol. 2019;1122:41-58.
[8] YAMAGUCHI M, HIRAI S, TANAKA Y, et al. Pericyte-myofibroblast transition in the human lung. Biochem Biophys Res Commun. 2020; 528(2):269-275.
[9] RICARD N, TU L, LE HIRESS M, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129(15):1586-1597.
[10] WILSON CL, STEPHENSON SE, HIGUERO JP, et al. Characterization of human PDGFR-β-positive pericytes from IPF and non-IPF lungs. Am J Physiol Lung Cell Mol Physiol. 2018;315(6):L991-L1002.
[11] BORDENAVE J, TU L, BERREBEH N, et al. Lineage Tracing Reveals the Dynamic Contribution of Pericytes to the Blood Vessel Remodeling in Pulmonary Hypertension. Arterioscler Thromb Vasc Biol. 2020;40(3): 766-782.
[12] HUNG CF, MITTELSTEADT KL, BRAUER R, et al. Lung pericyte-like cells are functional immune sentinel cells. Am J Physiol Lung Cell Mol Physiol. 2017;312(4):L556-L567.
[13] WU Y, LI P, GOODWIN AJ, et al. miR-145a Regulates Pericyte Dysfunction in a Murine Model of Sepsis. J Infect Dis. 2020;222(6):1037-1045.
[14] DUMPA V, BHANDARI V. Surfactant, steroids and non-invasive ventilation in the prevention of BPD. Semin Perinatol. 2018;42(7):444-452.
[15] 朱玥,卢红艳,郝晓波,等.SUMO 化修饰 C/EBPα在高氧暴露所致支气管肺发育不良早产大鼠中的动态表达及作用[J].中国当代儿科杂志,2018,20(5):68-74.
[16] EILKEN HM, DIÉGUEZ-HURTADO R, SCHMIDT I, et al. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun. 2017;8(1):1574.
[17] EHRENKRANZ RA, WALSH MC, VOHR BR, et al.Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353-1360.
[18] SCHMIDT AR, RAMAMOORTHY C. Bronchopulmonary dysplasia. Pediatr Anesth. 2022;32(2):174-180.
[19] CUMMINGS KW, BHALLA S. Pulmonary vascular diseases. Clin Chest Med. 2015;36(2):235-248, viii.
[20] DENG X, BAO Z, YANG X, et al. Molecular mechanisms of cell death in bronchopulmonary dysplasia. Apoptosis. 2022. doi: 10.1007/s10495-022-01791-4.
[21] KATO K, DIÉGUEZ-HURTADO R, PARK DY, et al. Pulmonary pericytes regulate lung morphogenesis. Nat Commun. 2018;9(1):2448.
[22] ARMULIK A, GENOVÉ G, BETSHOLTZ C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193-215.
[23] CRISAN M, YAP S, CASTEILLA L, et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell. 2008;3(3):301-313.
[24] RICARD N, TU L, LE HIRESS M, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129(15):1586.
[25] PAYNE LB, ZHAO H, JAMES CC, et al. The Pericyte Microenvironment during Vascular Development. Microcirculation. 2019;26(2):e12554.
[26] 蒋福林,艾冬青,官秋玥.周细胞概念及在血管形成信号转导通路研究中的进展[J].中国组织工程研究,2015,19(46):7504-7508.
[27] ALARCON-MARTINEZ L, YEMISCI M, DALKARA T. Pericyte morphology and function. Histol Histopathol. 2021;36(6):18314.
[28] CHI FH, WILSON CL, CHOW YH, et al. Effect of lung pericyte-like cell ablation on the bleomycin model of injury and repair. Am J Physiol. 2022;322(4):L607-L616.
[29] LIU X, ROWAN SC, LIANG J, et al. Categorization of Lung Mesenchymal Cells in Development and Fibrosis. iScience. 2021;24(6):102551.
[30] MATHEW R. Signaling Pathways Involved in the Development of Bronchopulmonary Dysplasia and Pulmonary Hypertension. Children (Basel). 2020;7(8):100.
[31] HUNG C, LINN G, CHOW YH, et al. Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis. Am J Respir Crit Care Med. 2013;188(7):820-830.
[32] 胡广志,卢红艳.肺周细胞与肺发育及肺部疾病研究进展[J].国际儿科学杂志,2022,49(3):197-201.
[33] SHAMMOUT B, JOHNSON JR. Pericytes in Chronic Lung Disease. Adv Exp Med Biol. 2019;1147:299-317.
[34] YUAN K, AGARWAL S, CHAKRABORTY A, et al. Lung Pericytes in Pulmonary Vascular Physiology and Pathophysiology. Compr Physiol. 2021;11(3):2227-2247.
[35] ZHANG Y, DONG X, SHIRAZI J, et al. Pulmonary endothelial cells exhibit sexual dimorphism in their response to hyperoxia. Am J Physiol Heart Circ Physiol. 2018;315(5):H1287-H1292.
[36] 王思思, 伍金林.高氧诱导肺血管内皮细胞损伤与支气管肺发育不良的研究进展[J]中华妇幼临床医学杂志(电子版),2021,17(3): 368-372.
[37] WU CF, CHIANG WC, LAI CF, et al. Transforming Growth Factor β-1 Stimulates Profibrotic Epithelial Signaling to Activate Pericyte-Myofibroblast Transition in Obstructive Kidney Fibrosis. Am J Pathol. 2013;182(1):118-131.
[38] YAO F, LUO Y, LIU YC, et al. Imatinib inhibits pericyte-fibroblast transition and inflammation and promotes axon regeneration by blocking the PDGF-BB/PDGFRβ pathway in spinal cord injury. Inflamm Regen. 2022;42(1):44.
[39] YANG T, GUO L, FANG Y, et al. Pericytes of Indirect Contact Coculture Decrease Integrity of Inner Blood-Retina Barrier Model In Vitro by Upgrading MMP-2/9 Activity. Dis Markers. 2021;2021:7124835.
[40] DONOGHUE L, TYBURSKI JG, STEFFES CP, et al. Vascular endothelial growth factor modulates contractile response in microvascular lung pericytes. Am J Surg. 2006;191(3):349-352.
[41] EDELMAN DA, JIANG Y, TYBURSKI J, et al. Pericytes and Their Role in Microvasculature Homeostasis. J Surg Res. 2006;135(2):305-311.
|