中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (33): 5376-5383.doi: 10.12307/2022.951
• 骨科植入物相关基础实验 Basic experiments of orthopedic implant • 上一篇 下一篇
经寰枕后间隙蛛网膜下腔注射是脊髓损伤模型大鼠可供选择的给药方式
李传鸿,俞 兴,杨永栋,杨凯坦,赵 赫
- 北京中医药大学东直门医院骨科,北京市 100700
Subarachnoid injection via the posterior atlanto-occipital interspace is an alternative way of administration in a rat model of spinal cord injury
Li Chuanhong, Yu Xing, Yang Yongdong, Yang Kaitan, Zhao He
- Department of Orthopedics, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China
摘要:
文题释义:
脊髓损伤动物模型:脊髓损伤动物模型被用于研究脊髓损伤的病理机制并探索最佳的干预措施。大鼠、小鼠、犬、兔是常见的实验对象,损伤部位多选择胸段脊髓。根据损伤时限的不同可分为急性机械性损伤与慢性压迫性损伤,前者又因不同的造模方式而分为挫伤、挤压伤、完全或部分横断损伤及牵拉/脱位损伤等,此外,还有缺血再灌注损伤及化学损伤等致伤方式。
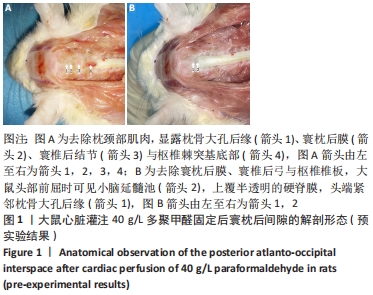
蛛网膜下腔注射:为避免血脑/脊髓屏障阻碍药物分子由血液进入脊髓组织,提高脑脊液中的药物浓度,通过蛛网膜下腔穿刺直接将药物注入蛛网膜下腔内的给药方式,常采用经皮穿刺腰椎棘突间隙、暴露硬脊膜直视下穿刺等方法进入蛛网膜下腔。蛛网膜下腔注射在动物实验中多用于镇痛研究,并作为中枢神经系统创伤、感染、退行性疾病等的干预途径。
背景:蛛网膜下腔给药是脊髓损伤动物实验中常用的干预方式,但目前缺乏可与缓释/控释剂型配合从而减少给药次数、避免蛛网膜下腔内置管的一次性较大剂量蛛网膜下腔内给药的方法。
目的:探索一种对于大鼠脊髓损伤模型可以一次性较大剂量给药的蛛网膜下腔注射方法。
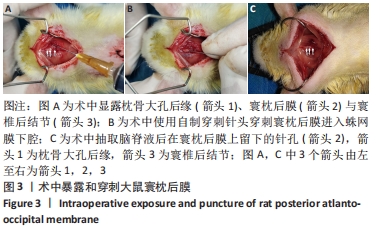
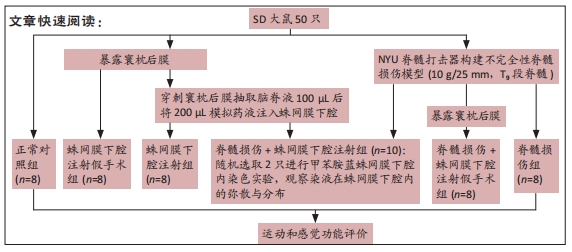
方法:将SD大鼠随机分为6组:脊髓损伤组采用NYU(New York University)脊髓打击器构建大鼠脊髓损伤模型,蛛网膜下腔注射组经寰枕后间隙将200 μL模拟药液(生理盐水)注入蛛网膜下腔,脊髓损伤+蛛网膜下腔注射组在脊髓损伤造模后进行蛛网膜下腔注射,正常对照组无处理,蛛网膜下腔注射假手术组暴露寰枕后膜后关闭切口,脊髓损伤+蛛网膜下腔注射假手术组在脊髓损伤造模后重复蛛网膜下腔注射假手术组的操作。借助甲苯胺蓝染色探索药物注射1.5 h后在蛛网膜下腔内的弥散与分布情况,通过旷场试验、斜板试验、热板试验、体质量测量等验证是否发生蛛网膜下腔注射相关并发症。
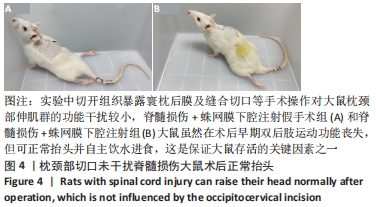
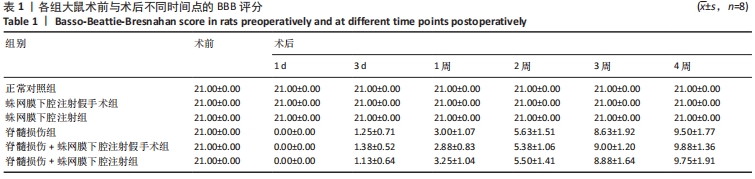
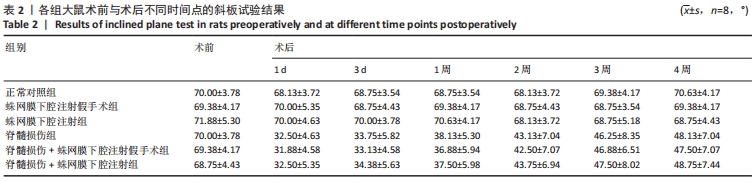
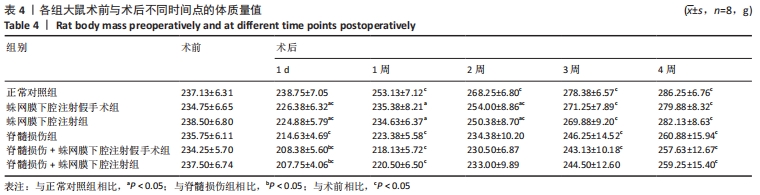
结果与结论:①各组经寰枕后间隙蛛网膜下腔注射未造成大鼠BBB评分、热板试验与斜板试验结果的异常。②蛛网膜下腔注射假手术组、蛛网膜下腔注射组大鼠术后1周时可恢复术前体质量(P > 0.05),脊髓损伤组、脊髓损伤+蛛网膜下腔注射假手术组、脊髓损伤+蛛网膜下腔注射组术后2周时体质量可恢复到术前水平(P > 0.05)。③术中及术后未观察到蛛网膜下腔注射引起大鼠中枢神经系统损伤的特殊征象,未见脑脊液/模拟药液外漏,术后大鼠可正常抬头,未出现精神萎靡和自残行为,蛛网膜下腔注射假手术组、蛛网膜下腔注射组未发生排尿排便障碍。④蛛网膜下腔注射后1.5 h,甲苯胺蓝溶液在大鼠蛛网膜下腔内广泛弥散,全段脊髓均有分布。⑤上述数据表明,经寰枕后间隙蛛网膜下腔注射的方法安全可靠,对脊髓损伤大鼠单次给药剂量200 μL时,药物能快速到达脊髓损伤部位,引起严重并发症的风险较小,因此是大鼠脊髓损伤模型可供选择的蛛网膜下腔给药方式。
https://orcid.org/0000-0002-1007-6127 (李传鸿) ;https://orcid.org/0000-0003-3598-3492 (俞兴)
中国组织工程研究杂志出版内容重点:人工关节;骨植入物;脊柱;骨折;内固定;数字化骨科;组织工程
中图分类号: