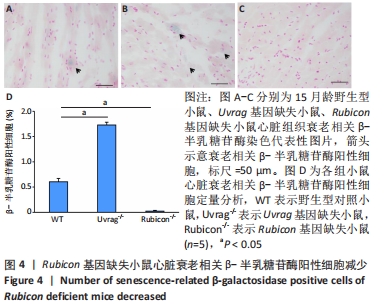
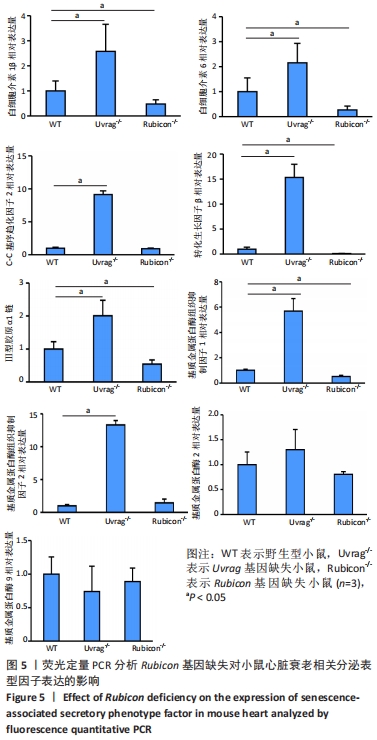
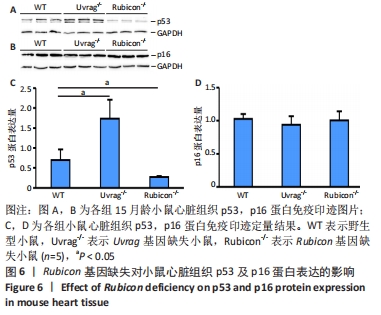
[1] FLORA GD, NAYAK MK. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr Pharm Des. 2019;25(38):4063-4084.
[2] SHAKERI H, LEMMENS K, GEVAERT AB, et al. Cellular Senescence Links Aging and Diabetes in Cardiovascular Disease. Am J Physiol Heart Circ Physiol. 2018;315(3): H448-H462.
[3] CALCINOTTO A, KOHLI J, ZAGATO E, et al. Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev. 2019;99(2):1047-1078.
[4] COLLADO M, SERRANO M. The Power and the Promise of Oncogene-induced Senescence Markers. Nat Rev Cancer. 2006;6(6):472-476.
[5] HERNANDEZ-SEGURA A, NEHME J, DEMARIA M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28(6):436-453.
[6] MIJIT M, CARACCIOLO V, MELILLO A, et al. Role of P53 in the Regulation of Cellular Senescence. Biomolecules. 2020;10(3):420.
[7] ZHU P, ZHANG C, GAO Y, et al. The Transcription Factor Slug Represses P16(Ink4a) and Regulates Murine Muscle Stem Cell Aging. Nat Commun. 2019;10(1):2568.
[8] ZHU Y, TCHKONIA T, PIRTSKHALAVA T, et al. The Achilles’ Heel of Senescent Cells: from Transcriptome to Senolytic Drugs. Aging Cell. 2015;14(4):644-658.
[9] WALASZCZYK A, DOOKUN E,REDGRAVE R, et al. Pharmacological Clearance of Senescent Cells Improves Survival and Recovery in Aged Mice Following Acute Myocardial Infarction. Aging Cell. 2019;18(3):e12945.
[10] SHIMIZU I, MINAMINO T. Cellular Senescence in Cardiac Diseases. J Cardiol. 2019; 74(4):313-319.
[11] WONG SQ, KUMAR AV, MILLS J, et al. Autophagy in Aging and Longevity. Hum Genet. 2020;139(3):277-290.
[12] GUO F,LIU X,CAI H, et al. Autophagy in Neurodegenerative Diseases:Pathogenesis and Therapy. Brain Pathol. 2018;28(1):3-13.
[13] LUO F, SANDHU AF, RUNGRATANAWANICH W, et al. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int J Mol Sci. 2020;21(19):7174.
[14] ALLAIRE M, RAUTOU PE, CODOGNO P, et al. Autophagy in Liver Diseases: Time for Translation? J Hepatol. 2019;70(5):985-998.
[15] KE PY. Mitophagy in the Pathogenesis of Liver Diseases. Cells. 2020;9(4):831.
[16] DU J, LIU Y, FU J. Autophagy and Heart Failure. Adv Exp Med Biol. 2020;1207:223-227.
[17] CORSETTI G, PASINI E, ROMANO C, et al. How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives. Int J Mol Sci. 2021;22(7):3332.
[18] YOUNG A, NARITA R, FERREIRA M, et al. Autophagy Mediates the Mitotic Senescence Transition. Genes Dev. 2009;23(7):798-803.
[19] KWON Y, KIM JW, JEOUNG JA, et al. Autophagy Is Pro-Senescence When Seen in Close-Up, but Anti-Senescence in Long-Shot. Mol Cells. 2017;40(9):607-612.
[20] PULAKAT L, CHEN HH. Pro-Senescence and Anti-Senescence Mechanisms of Cardiovascular Aging: Cardiac MicroRNA Regulation of Longevity Drug-Induced Autophagy. Front Pharmacol. 2020;11:774.
[21] ZHONG Y, WANG QJ, LI XT, et al. Distinct Regulation of Autophagic Activity by Atg14L and Rubicon Associated with Beclin 1-phosphatidylinositol-3-kinase Complex. Nature Cell Biol. 2009;11(4):468-476.
[22] MATSUNAGA K, SAITOH T, TABATA K, et al. Two Beclin 1-binding Proteins, Atg14L and Rubicon,Reciprocally Regulate Autophagy at Different Stages. Nat Cell Biol. 2009;11(4):385-396.
[23] KANG R, ZEH HJ, LOTZE MT, et al. The Beclin 1 Network Regulates Autophagy and Apoptosis. Cell Death and Differentiation. 2011;18(4):571-580.
[24] SUN, QM, ZHANG J, FAN, WL, et al. The RUN Domain of Rubicon Is Important for hVps34 Binding, Lipid Kinase Inhibition, and Autophagy Suppression. Journal of Biological Chemistry. 2011;286(1):185-191.
[25] SUN QM, ZHANG J, FAN WL, et al. Rubicon Controls Endosome Maturation as A Rab7 Effector. Proc Natl Acad Sci U S A. 2010;107(45):19338-19343.
[26] NAKAMURA S, OBA M, SUZUKI M, et al. Suppression of Autophagic Activity by Rubicon is A Signature of Aging. Nat Commun. 2019;10(1):847.
[27] SONG Z, AN L, YE Y, et al. Essential Role for UVRAG in Autophagy and Maintenance of Cardiac Function. Cardiovasc Res. 2014;101(1):48-56.
[28] 来帅威,张沙沙,刘晓芸,等. 心脏细胞衰老与Uvrag基因的缺失[J]. 中国组织工程研究,2021,25(14):2241-2246.
[29] ZI Z, SONG Z, ZHANG S, et al. Rubicon Deficiency Enhances Cardiac Autophagy and Protects Mice from Lipopolysaccharide-Induced Lethality and Reduction in Stroke Volume. J Cardiovasc Pharmacol. 2015;65(3):252-261.
[30] THOMAS N, GURVICH C, KULKARNI J. Sex Differences in Aging and Associated Biomarkers. Adv Exp Med Biol. 2019;1178:57-76.
[31] DODIG S, CEPELAK I, PAVIC I. Hallmarks of Senescence and Aging. Biochem Med (Zagreb). 2019;29(3):030501.
[32] ZHANG J, CHENG P, PU K. Recent Advances of Molecular Optical Probes in Imaging of beta-Galactosidase. Bioconjug Chem. 2019;30(8):2089-2101.
[33] LOPES-PACIENCIA S, SAINT-GERMAIN E, ROWELL MC, et al. The Senescence-associated Secretory Phenotype and its Regulation. Cytokine. 2019;117:15-22.
[34] HOSHINO A, MITA Y, OKAWA Y, et al. Cytosolic P53 Inhibits Parkin-Mediated Mitophagy and Promotes Mitochondrial Dysfunction in The Mouse Heart. Nat Commun. 2013;4:2308.
[35] REN X, CHEN L, XIE J, et al. Resveratrol Ameliorates Mitochondrial Elongation via Drp1/Parkin/PINK1 Signaling in Senescent-Like Cardiomyocytes. Oxid Med Cell Longev. 2017;2017:4175353.
[36] MADEO F, TAVERNARAKIS N, KROEMER G. Can autophagy promote longevity? Nat Cell Biol. 2010;12(9):842-846.
[37] FERNÁNDEZ ÁF, SEBTI S, WEI Y, et al. Disruption of The Beclin 1-BCL2 Autophagy Regulatory Complex Promotes Longevity in Mice. Nature. 2018;558(7708):136-140.
[38] XU S, CAI Y, WEI Y. mTOR Signaling from Cellular Senescence to Organismal Aging. Aging Dis. 2014;5(4):263-273.
[39] NISHIMURA A, SHIMAUCHI T, TANAKA T, et al. Hypoxia-Induced Interaction of Filamin with Drp1 Causes Mitochondrial Hyperfission-Associated Myocardial Senescence. Sci Signal. 2018;11(556):eaat5185.
[40] ANDERSON R, LAGNADO A, MAGGIORANI D, et al. Length-Independent Telomere Damage Drives Post-Mitotic Cardiomyocyte Senescence. EMBO J. 2019;38(5): e100492.
[41] LIU XY, ZHANG S, AN L, et al. Loss of Rubicon Ameliorates Doxorubicin-Induced Cardiotoxicity through Enhancement of Mitochondrial Quality. Int J Cardiol. 2019; 296:129-135.
|