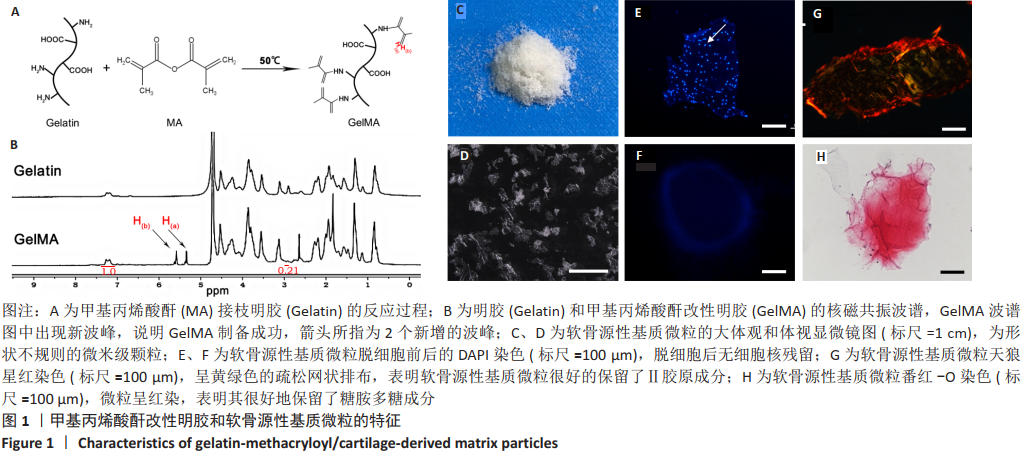
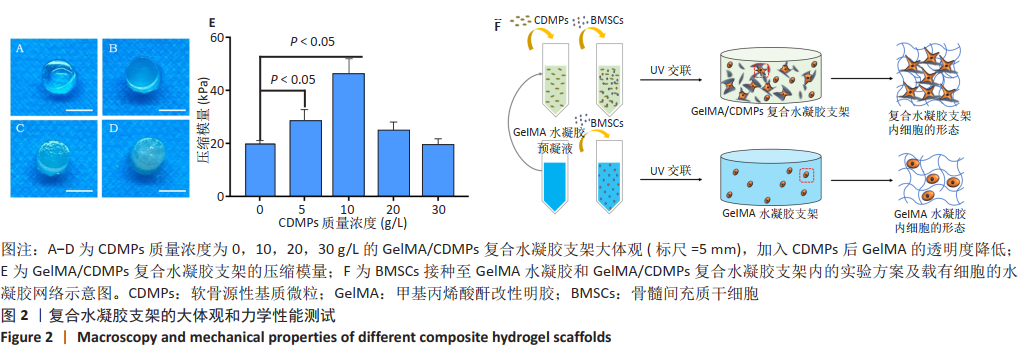
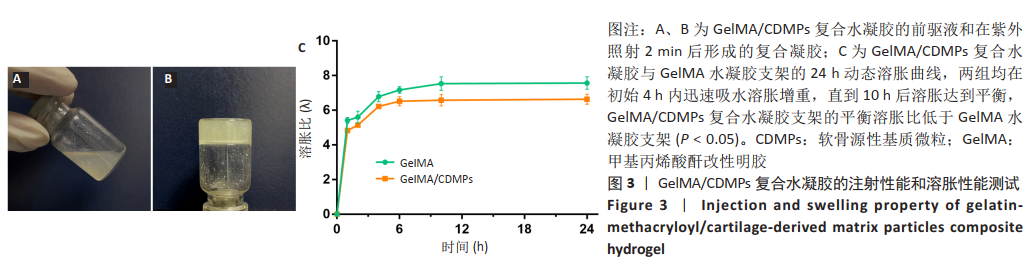
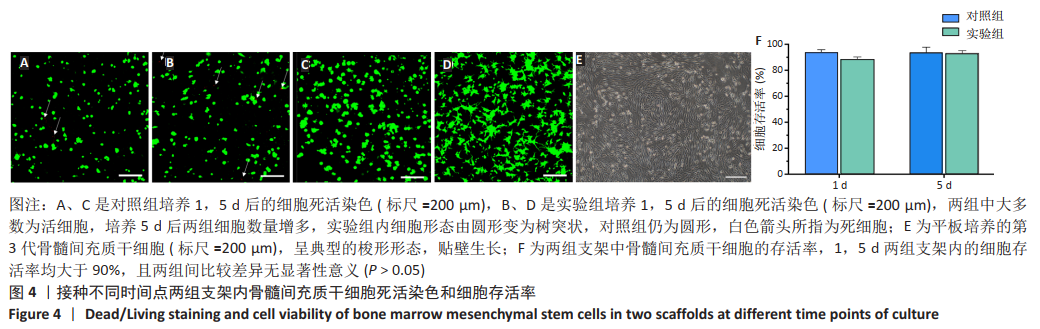
[1] MADEIRA C, SANTHAGUNAM A, SALGUEIRO JB, et al. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015;33(1): 35-42.
[2] MAKRIS EA, GOMOLL AH, MALIZOS KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21-34.
[3] 刘奕,谢林.组织工程技术修复损伤关节软骨的研究与应用[J].中国组织工程研究,2013,17(41):7310-7316.
[4] CAI L, DEWI RE, HEILSHORN SC. Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Adv Funct Mater. 2015;25(9):1344-1351.
[5] LI J, CHEN G, XU X, et al. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen Biomater. 2019;6(3):129-140.
[6] VEGA SL, KWON MY, BURDICK JA. Recent advances in hydrogels for cartilage tissue engineering. Eur Cell Mater. 2017;33:59-75.
[7] PUPKAITE J, ROSENQUIST J, HILBORN J, et al. Injectable Shape-Holding Collagen Hydrogel for Cell Encapsulation and Delivery Cross-linked Using Thiol-Michael Addition Click Reaction. Biomacromolecules. 2019;20(9): 3475-3484.
[8] YING G, JIANG N, PARRA-CANTU C, et al. Bioprinted Injectable Hierarchically Porous Gelatin Methacryloyl Hydrogel Constructs with Shape-Memory Properties. Adv Funct Mater. 2020;30(46):2003740.
[9] SUN M, SUN X, WANG Z, et al. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers (Basel). 2018;10(11):1290.
[10] SPILLER KL, MAHER SA, LOWMAN AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. 2011;17(4):281-299.
[11] NICHOL JW, KOSHY ST, BAE H, et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536-5544.
[12] WANG Y, MA M, WANG J, et al. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials (Basel, Switzerland). 2018;11(8):1345.
[13] SHIN SR, BAE H, CHA JM, et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano. 2012;6(1):362-372.
[14] SHIN H, OLSEN BD, KHADEMHOSSEINI A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials. 2012;33(11):3143-3152.
[15] ZHU W, CUI H, BOUALAM B, et al. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology. 2018;29(18):185101.
[16] SHIN SR, ZIHLMANN C, AKBARI M, et al. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small. 2016; 12(27):3677-3689.
[17] NAVAEI A, SAINI H, CHRISTENSON W, et al. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016;41:133-146.
[18] 肖统光,张一民,郭维民,等.细胞外基质来源支架在软骨组织工程中的应用[J].中国组织工程研究,2016,20(38):5737-5744.
[19] YIN H, WANG Y, SUN X, et al. Functional tissue-engineered microtissue derived from cartilage extracellular matrix for articular cartilage regeneration. Acta Biomater. 2018;77:127-141.
[20] YIN H, WANG Y, SUN Z, et al. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 2016;33:96-109.
[21] ALMEIDA HV, ESWARAMOORTHY R, CUNNIFFE GM, et al. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016;36:55-62.
[22] CHANG CH, CHEN CC, LIAO CH, et al. Human acellular cartilage matrix powders as a biological scaffold for cartilage tissue engineering with synovium-derived mesenchymal stem cells. J Biomed Mater Res A. 2014; 102(7):2248-2257.
[23] SUTHERLAND AJ, DETAMORE MS. Bioactive Microsphere-Based Scaffolds Containing Decellularized Cartilage. Macromol Biosci. 2015;15(7):979-989.
[24] ABRAMS GD, MALL NA, FORTIER LA, et al. BioCartilage: Background and Operative Technique. Oper Tech Sports Med. 2013;21(2):116-124.
[25] LOESSNER D, MEINERT C, KAEMMERER E, et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat Protoc. 2016;11(4):727-746.
[26] 杨俊丽,韩霞,孙明启,等.兔骨髓间充质干细胞的生物学特征及原代培养[J].中国组织工程研究,2015,19(50):8043-8047.
[27] ROTHRAUFF BB, SHIMOMURA K, GOTTARDI R, et al. Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix. Acta Biomater. 2017;49:140-151.
[28] KILIC BEKTAS C, HASIRCI V. Mimicking corneal stroma using keratocyte-loaded photopolymerizable methacrylated gelatin hydrogels. J Tissue Eng Regen Med. 2018;12(4):e1899-e1910.
[29] PEPELANOVA I, KRUPPA K, SCHEPER T, et al. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering (Basel, Switzerland). 2018;5(3):55.
[30] YUE K, TRUJILLO-DE SANTIAGO G, ALVAREZ MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-271.
[31] VISSER J, GAWLITTA D, BENDERS KE, et al. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174-182.
[32] GU L, LI T, SONG X, et al. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen Biomater. 2020;7(2):195-202.
[33] FRANTZ C, STEWART KM, WEAVER VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195-4200.
[34] FERNANDES H, MORONI L, VAN BLITTERSWIJK C, et al. Extracellular matrix and tissue engineering applications. J Mater Chem. 2009;19(31):5474-5484.
[35] LIU Y, CHAN-PARK MB. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials. 2010;31(6):1158-1170. |
 文题释义:
文题释义: