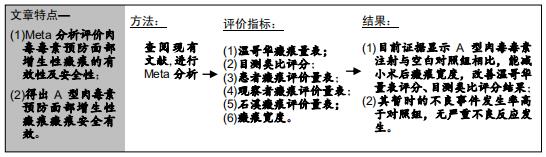
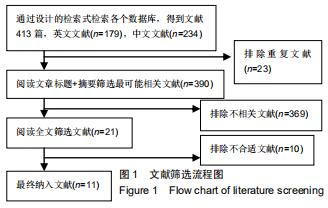
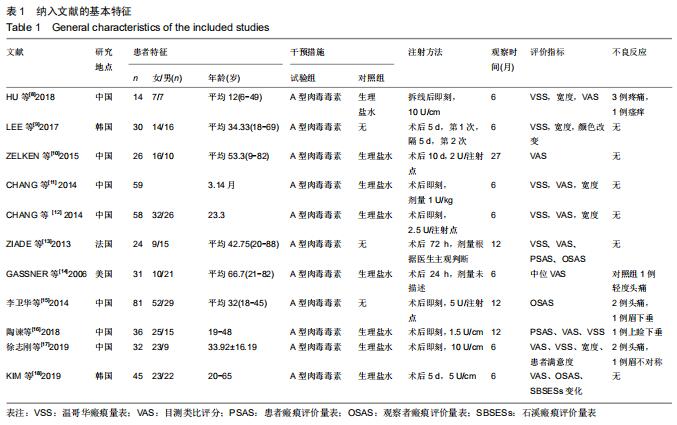
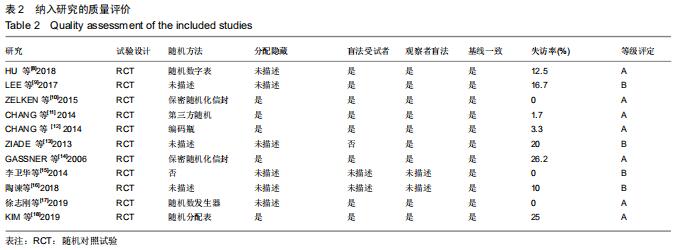
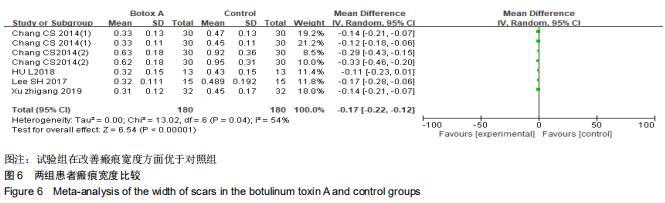
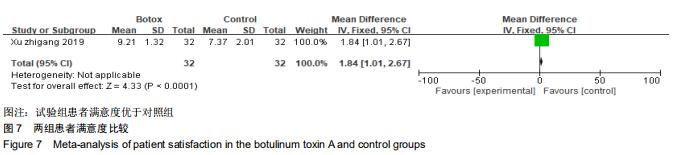
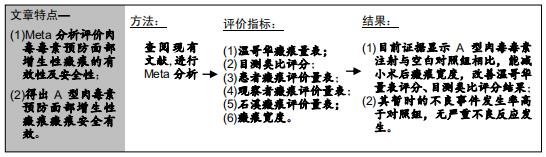
|
[1] BIJLARD E, KOUWENBERG CA,TIMMAN R, et al. Burden of keloid disease: a cross-sectional health-related qualit y of life assessment. Acta Derm Venereol.2017;97(2):225-229.
[2] OOSTERWIJK AM, MOUTON LJ, SCHOUTEN H, et al. Prevalence of scar contractures after burn: a systematic review.Burns.2017;43(1): 41-49.
[3] MARTINS JMP, OLIVEIRA FDS, LIMA EOC, et al. Use of derived adipose stem cells to reduce complications of c utaneous scarring in smokers. An experimental model in rats.Acta Cir Bras.2019;34(6): e201900605.
[4] MENEZES MCS, VASCONCELLOS LS, NUNES CB, et al. Evaluation of the use of tacrolimus ointment for the preve ntion of hypertrophic scars in experimental model.An Bras Dermatol.2019;94(2):164-171.
[5] 于水,王雅文,赵梓纲.A 型肉毒素在皮肤科的应用现状和前景[J].中国皮肤性病学杂志,2018,32(1):101-104.
[6] KASYANJU CARRERO LM, MA WW, LIU HF, et al. Botulinum toxin type A for the treatment and prevention of h ypertrophic scars and keloids: Updated review.J Cosmet Dermatol.2019;18(1):10-15.
[7] PRODROMIDOU A, FROUNTZAS M, VLACHOS DE, et al. Botulinum toxin for the prevention and healing of woun d scars: A systematic review of the literature.Plast Surg.2015;23(4): 260-264.
[8] HU L, ZOU Y, CHANG SJ, et al. Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial.Plast Reconstr Surg.2018;141(3):646-650.
[9] LEE SH, MIN HJ, KIM YW, et al. The efficacy and safety of early postoperative botulinum toxin A injectio n for facial scars.Aesthet Plast Surg.2018;42(2):530-537.
[10] ZELKEN J, YANG SY, CHANG CS, et al. Donor site aesthetic enhancement with preoperative botulinum toxin in f orehead flap nasal reconstruction.Ann Plast Surg.2016;77(5):535-538.
[11] CHANG CS, WALLACE CG, HSIAO YC, et al.Botulinum toxin to improve results in cleft lip repair. Plast Reco nstr Surg.2014;134(3):511-516.
[12] CHANG CS, WALLACE CG, HSIAO YC, et al. Botulinum toxin to improve results in cleft lip repair: a doubleblind ed, randomized, vehicle-controlled clinical trial.PLoS ONE.2014;9(12):e115690.
[13] ZIADE M, DOMERGUE S, BATIFOL D, et al. Use of botulinum toxin type A to improve treatment of facial woun ds: a prospective randomised study.J Plast Reconstr Aesthet Surg.2013;66(2):209-214.
[14] GASSNER HG, BRISSETT AE, OTLEY CC, et al. Botulinum toxin to improve facial wound healing: a prospective, blinded, placebo-controlled study.Mayo Clin Proc.2006;81(8):1023-1028.
[15] 李卫华,高玉伟,孙志成.A型肉毒毒素在面部直线形瘢痕修复术中的应用[J].中国美容整形外科杂志,2014,25(7):426-429.
[16] 陶谏,刘宾,汪阳,等.A 型肉毒毒素对减轻额部术后瘢痕增生的临床研究[J].陕西医学杂志,2018,47(8):1011-1013.
[17] 徐志刚,胡大海.A 型肉毒毒素减轻面部整形切口瘢痕的临床观察[J].安徽医药,2019,23(5):1010-1013.
[18] KIM SH, LEE SJ, LEE JW, et al. Clinical trial to evaluate the efficacy of botulinum toxin type A injection for reducing scars in patients with forehead laceration: A double-blinded, randomized controlled study. Medicine (Baltimore).2019;98(34):e16952.
[19] LI YH, YANG J, LIU JQ, et al. A Randomized, Placebo-Controlled, Double-Blind, Prospective Clinical Trial of B otulinum Toxin Type A in Prevention of Hypertrophic Scar Development in Median Sternotomy Wound.A esthetic Plast Surg.2018;42(5):1364-1369.
[20] KIM YS, LEE HJ, CHO SH, et al. Early postoperative treatment of thyroidectomy scars using botulinum toxin: a split-scar, double-blind randomized controlled trial.Wound Repair Regen.2014;22(5):605-612.
[21] 王小玉,王小琴,刘琰,等.面部小面积瘢痕手术切口早期应用A型肉毒毒素注射联合强脉冲光照射的临床效果[J].实用皮肤病学杂志,2013,6(5): 271-273.
[22] 杨萍,张子玉.A型肉毒毒素联合减张压迫法于面部整形切口愈合应用中的临床分析[J].中外女性健康研究,2017,18:59-64.
[23] 汪阳,张颖,田婧.局部注射 A 型肉毒毒素对额颞部良性肿瘤切除术后切口瘢痕增生的防治作用[J].河北医学, 2017,23(3):486-489.
[24] 杨瑞年,李明,吴承道.面部整形美容切口中A型肉毒毒素联合减张压迫法应用研究[J].中国医疗美容,2014,(6);3.
[25] 卫东.Botox A 早期切口内注射联合减张法对面部小面积瘢痕手术切口恢复效果的影响[J].医学研究杂志,2017,46(6):133-136.
[26] 刘涛,杨德发.A 型肉毒毒素+减张压迫法用于面部整形美容切口中的研究[J].中国医疗美容,2017,7(5):7-9.
[27] WILSON AM. Use of botulinum toxin type A to prevent widening of facial scars.Plast Reconstr Surg.2006;117(6):1758-1766.
[28] XIAO Z, ZHANG F, CUI Z. Treatment of hypertrophic scars with intralesional botulinum toxin type A injections: a preliminary report. Aesthetic Plast Surg.2009;33(3):409-412.
[29] GASSNER HG, SHERRIS DA, OTLEY CC. Treatment of facial wounds with botulinum toxin A improves cosmetic o utcome in primates.Plast Reconstr Surg.2000;105(6):1948-1953; discussion 1954-5.
[30] SHERRIS DA, GASSNER HG. Botulinum toxin to minimize facial scarring.Facial Plast Surg.2002;18(1):35-39.
[31] PRODROMIDOU A, FROUNTZAS M, VLACHOS DE, et al. Botulinum toxin for the prevention and healing of wound sca rs: A systematic review of the literature.Plast Surg.2015;23(4):260-264.
[32] ZHANG DZ, LIU XY, XIAO WL, et al. Botulinum Toxin Type A and the Prevention of Hypertrophic Scars on t he Maxillofacial Area and Neck: A Meta-Analysis of Randomized Controlled Trials. PLoS One.2016; 11(3): e0151627.
[33] WANG Y, WANG J, ZHANG J, et al. Effectiveness and Safety of Botulinum Toxin Type A Injection for Scar Preve ntion: A Systematic Review and Meta-analysis.AestheticPlast Surg.2019;43(5):1241-1249.
[34] 常宁,李广帅,刘林嶓,等.术后行肉毒毒素注射对切口愈合效果影响的Meta分析[J].中国美容医学,2015;24 (23):17-20.
[35] GILLANDERS SL, ANDERSON S, MELLON L, et al. A systematic review and Meta-analysis: Do absorbable or non-abso rbable suture materials differ in cosmetic outcomes in patients requiring primary closure of facial wounds? J Plast Reconstr Aesthet Surg.2018;71(12): 1682-1692.
[36] COHEN JL, SCUDERI N. Safety and Patient Satisfaction of AbobotulinumtoxinA for Aesthetic Use: A Systematic Review.Aesthet Surg J.2017;37(suppl_1):S32-S44.
|