| [1]Shaw I, Rider S, Mullins J, et al. Pericytes in the renal vasculature: roles in health and disease. Nat Rev Nephrol. 2018;14(8):521-534.[2]Yamazaki T, Mukouyama YS. Tissue Specific Origin, Development, and Pathological Perspectives of Pericytes. Front Cardiovasc Med. 2018;5:78.[3]Bodnar RJ, Satish L, Yates CC, et al. Pericytes: A newly recognized player in wound healing. Wound Repair Regen. 2016;24(2):204-214.[4]Caporali A, Martello A, Miscianinov V, et al. Contribution of pericyte paracrine regulation of the endothelium to angiogenesis. Pharmacol Ther. 2017;171:56-64.[5]陈俊敏,张祥建,刘晓霞,等.周细胞与中枢神经系统疾病[J].中国卒中杂志, 2018,13(1):90-95.[6]毛鑫羽,马惠宁,徐士欣,等.周细胞在缺血性心脏病病理环节中的研究进展[J]. 中华老年心脑血管病杂志, 2016,18(7):756-758.[7]Caplan AI. New MSC: MSCs as pericytes are Sentinels and gatekeepers. J Orthop Res. 2017;35(6):1151-1159.[8]Mravic M, Asatrian G, Soo C, et al. From pericytes to perivascular tumours: correlation between pathology, stem cell biology, and tissue engineering. Int Orthop. 2014;38(9):1819-1824.[9]张弘,张志光.血管周细胞的研究进展[J].国际口腔医学杂志, 2013,40(4): 529-532.[10]Harrell CR, Simovic Markovic B, et al. Molecular mechanisms underlying therapeutic potential of pericytes. J Biomed Sci. 2018;25(1): 21.[11]Trost A, Schroedl F, Lange S, et al. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci. 2013;54(13): 7910-7921.[12]Reyahi A, Nik AM, Ghiami M, et al. Foxf2 Is Required for Brain Pericyte Differentiation and Development and Maintenance of the Blood-Brain Barrier. Dev Cell. 2015;34(1):19-32.[13]Trost A, Lange S, Schroedl F, et al. Brain and Retinal Pericytes: Origin, Function and Role. Front Cell Neurosci. 2016;10:20.[14]Dias Moura Prazeres PH, Sena IFG, Borges IDT, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol. 2017; 427(1):6-11.[15]Prazeres PHDM, Almeida VM, Lousado L, et al. Macrophages Generate Pericytes in the Developing Brain. Cell Mol Neurobiol. 2018; 38(4):777-782.[16]Mangialardi G, Cordaro A, Madeddu P. The bone marrow pericyte: an orchestrator of vascular niche. Regen Med. 2016;11(8):883-895.[17]Schrimpf C, Teebken OE, Wilhelmi M, et al. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014;51(4):247-258.[18]Fernández-Klett F, Priller J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J Cereb Blood Flow Metab. 2015; 35(6):883-887.[19]van Dijk CG, Nieuweboer FE, Pei JY, et al. The complex mural cell: pericyte function in health and disease. Int J Cardiol. 2015;190:75-89.[20]Ahmed TA, El-Badri N. Pericytes: The Role of Multipotent Stem Cells in Vascular Maintenance and Regenerative Medicine. Adv Exp Med Biol. 2018;1079:69-86.[21]Stallcup WB, You WK, Kucharova K, et al. NG2 Proteoglycan- Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression. Microcirculation. 2016;23(2):122-133.[22]Borysova L, Dora KA. The three faces of pericytes. J Physiol. 2018; 596(16):3453-3454.[23]Sá da Bandeira D, Casamitjana J, Crisan M. Pericytes, integral components of adult hematopoietic stem cell niches. Pharmacol Ther. 2017;171:104-113.[24]张磊,吴溯帆.周细胞的研究进展[J].中国美容整形外科杂志, 2017(7): 441-443.[25]徐立霞, 武俏丽.周细胞与阿尔兹海默病的相关研究进展[J]. 继续医学教育, 2018,28(7):127-129.[26]李芮琳,胡利民,王少峡,等.周细胞在脑血管疾病中的细胞功能研究现状[J].中国临床药理学杂志, 2018,34(3):390-392.[27]Hung CF, Mittelsteadt KL, Brauer R, et al. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol. 2017;312(4):L556-L567.[28]Guimarães-Camboa N, Cattaneo P, Sun Y, et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell. 2017;20(3):345-359.e5.[29]Xu J, Gong T, Heng BC, et al. A systematic review: differentiation of stem cells into functional pericytes. FASEB J. 2017;31(5):1775-1786.[30]Underly RG, Levy M, Hartmann DA, et al. Pericytes as Inducers of Rapid, Matrix Metalloproteinase-9-Dependent Capillary Damage during Ischemia. J Neurosci. 2017;37(1):129-140.[31]Murray IR, Péault B. Q&A: Mesenchymal stem cells - where do they come from and is it important. BMC Biol. 2015;13:99.[32]De Souza LE, Malta TM, Kashima Haddad S, et al. Mesenchymal Stem Cells and Pericytes: To What Extent Are They Related. Stem Cells Dev. 2016;25(24):1843-1852.[33]Hardy WR, Moldovan NI, Moldovan L, et al. Transcriptional Networks in Single Perivascular Cells Sorted from Human Adipose Tissue Reveal a Hierarchy of Mesenchymal Stem Cells. Stem Cells. 2017; 35(5):1273-1289.[34]Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313.[35]Corselli M, Chin CJ, Parekh C, et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121(15):2891-2901.[36]Fitzsimmons REB, Mazurek MS, Soos A, et al. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018;2018:8031718.[37]Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30(6): 1104-1109.[38]Feng J, Mantesso A, De Bari C, et al. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A. 2011;108(16):6503-6508.[39]König MA, Canepa DD, Cadosch D, et al. Direct transplantation of native pericytes from adipose tissue: A new perspective to stimulate healing in critical size bone defects. Cytotherapy. 2016;18(1):41-52.[40]Murray IR, Baily JE, Chen WCW, et al. Skeletal and cardiac muscle pericytes: Functions and therapeutic potential. Pharmacol Ther. 2017; 171:65-74.[41]Park TS, Gavina M, Chen CW, et al. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011;20(3):451-463.[42]Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255-267.[43]Chen CW, Okada M, Proto JD, et al. Human pericytes for ischemic heart repair. Stem Cells. 2013;31(2):305-316.[44]Chen WC, Baily JE, Corselli M, et al. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells. 2015;33(2):557-573.[45]Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327-334.[46]Wei Q, Frenette PS. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity. 2018;48(4):632-648.[47]Itkin T, Gur-Cohen S, Spencer JA, et al. Corrigendum: Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;538(7624):274.[48]Pinho S, Lacombe J, Hanoun M, et al. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7): 1351-1367.[49]Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324-336.[50]He Q, Scott Swindle C, Wan C, et al. Enhanced Hematopoietic Stem Cell Self-Renewal-Promoting Ability of Clonal Primary Mesenchymal Stromal/Stem cells Versus Their Osteogenic Progeny. Stem Cells. 2017;35(2):473-484.[51]Butko E, Pouget C, Traver D. Complex regulation of HSC emergence by the Notch signaling pathway. Dev Biol. 2016;409(1):129-138.[52]Harkness L, Zaher W, Ditzel N, et al. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res Ther. 2016;7:4.[53]Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227-230.[54]Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977-988.[55]Tzeng YS, Li H, Kang YL, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117(2):429-439.[56]Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013; 495(7440):231-235.[57]Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829-834.[58]Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473): 637-643.[59]Han TJ, Wang X. Leptin and its receptor in hematologic malignancies. Int J Clin Exp Med. 2015;8(11):19840-19849.[60]Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab. 2015;33(5):474-485.[61]Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154-168.[62]Hu X, Garcia M, Weng L, et al. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat Commun. 2016;7:13095.[63]Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891-903.[64]Zhou BO, Ding L, Morrison SJ. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1.Elife. 2015;4:e05521. |
.jpg)
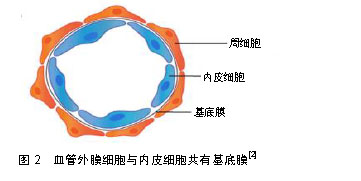
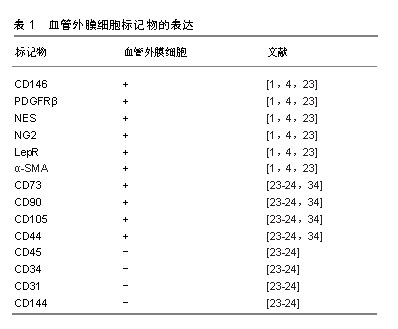
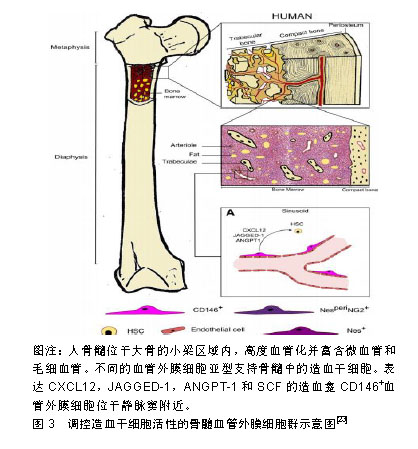
.jpg)
.jpg)