| [1]Andersen OZ,Offermanns V,Sillassen M,et al.Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials. 2013;34(24):5883-5890.[2]Ferraris S,Spriano S.Antibacterial titanium surfaces for medical implants. Mater Sci Eng C Mater Biol Appl.2016;61: 965-978.[3]Feng YF,Wang L,Zhang Y,et al.Effect of reactive oxygen species overproduction on osteogenesis of porous titanium implant in the present of diabetes mellitus, Biomaterials. 2013;34(9):2234-2243.[4]Andersen OZ,Offermanns V,Sillassen M,at al.Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials. 2013;34(24):5883-5890.[5]Low KL,Tan SH,Zein SH,et al.Calcium phosphate-based composites as injectable bone substitute materials.J Biomed Mater Res B Appl Biomater.2010;94(1):273-286.[6]Raphel J,Holodniy M,Goodman SB,et al.Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants.Biomaterials. 2016;84:301-314.[7]Campoccia D,Montanaro L,Arciola CR.A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials. 2013;34(33):8018-8029.[8]Goodman SB,Yao Z,Keeney M,et al.The future of biologic coatings for orthopaedic implants.Biomaterials. 2013;34(13): 3174-3183.[9]Zhang BG,Myers D E,Wallace G G,et al.Choong Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings.Int J Mol Sci. 2014;15(7): 11878-11921.[10]Albrektsson T,Johansson C.Osteoinduction, osteoconduction and osseointegration. Eur Spine J.2001;10(2):S96-S101.[11]Ozawa S,Kasugai S.Evaluation of implant materials (hydroxyapatite, glass-ceramics, titanium) in rat bone marrow stromal cell culture.Biomaterials.1996;17(1):23-29.[12]Wang H,Eliaz N,Xiang Z,et al.Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy Biomaterials. 2006; 27(23):4192-4203. [13]Nair AK,Gautieri A,Chang SW,et al.Molecular mechanics of mineralized collagen fibrils in bone.Nat Commun.2013;4:1724.[14]Ghosh S,Wu V,Pernal S,et al.Self-Setting Calcium Phosphate Cements with Tunable Antibiotic Release Rates for Advanced Antimicrobial Applications.ACS Appl Mater Interfaces.2016; 8(12):7691-7708.[15]Ahmadi S,Mohammadi I,Sadrnezhaad SK.Hydroxyapatite based and anodic Titania nanotube biocomposite coatings: Fabrication, characterization and electrochemical behavior. Surf Coat Tech.2015;287:67-75.[16]Filova E,Fojt J,Kryslova M,et al.The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of Saos-2 cells.Int J Nanomedicine. 2015;10: 7145-7163.[17]Zhao L,Liu L,Wu Z,et al.Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation.Biomaterials. 2012;33(9):2629-2641.[18]Brammer KS,Oh S,Cobb CJ,et al.Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater.2009;5(8):3215-3223. [19]Brammer KS,Oh S,Frandsen CJ,at al.Nanotube surface triggers increased chondrocyte extracellular matrix production.Mater Sci Eng C Mater Biol Appl. 2010;30(4):518-525.[20]Park J,Bauer S,Schlegel KA,et al.TiO2 nanotube surfaces: 15nm—an optimal length scale of surface topography for cell adhesion and differentiation. Small.2009;5(6):666-671. [21]Xu Z,Lai Y,Wu D,et al.Increased Mesenchymal Stem Cell Response and Decreased Staphylococcus aureus Adhesion on Titania Nanotubes without Pharmaceuticals.Biomed Res Int.2015;2015:172898. [22]田昂,薛向欣,杨合,等.Ti02纳米管阵列在模拟体液中诱导nHA沉积行为的研究[J].功能材料,2013,44(2):166-171.[23]Yao C,Webster TJ.Prolonged antibiotic delivery from anodized nanotubular titanium using a co-precipitation drug loading method.J Biomed Mater Res B Appl Biomater. 2009; 91(2):587-595.[24]Heimann RB.Plasma-spray coating: principles and applications. John Wiley & Sons, 2007.[25]Li S,Huang B,Chen Y,et al.Hydroxyapatite-coated femoral stems in primary total hip arthroplasty: a meta-analysis of randomized controlled trials.Int J Surg.2013;11(6):477-482.[26]Munzinger U,Guggi T,Kaptein B,et al. A titanium plasma-sprayed cup with and without hydroxyapatite-coating: a randomized radiostereometric study of stability and osseointegration. Hip Int.2013;23(1):33-39.[27]Melton JT,Mayahi R,Baxter SE,et al.Long-term outcome in an uncemented,hydroxyapatite-coated total knee replacement: a 15-to 18-year survivorship analysis J Bone Joint Surg Br. 2012;94(8):1067-1070. [28]Narayanan R,Seshadri SK,Kwon TY,et al.Calcium phosphate-based coatings on titanium and its alloys.J Biomed Mater Res B Appl Biomater.2008;85(1):279-299.[29]Rajesh P,Muraleedharan CV,Komath M,et al.Laser surface modification of titanium substrate for pulsed laser deposition of highly adherent hydroxyapatite.J Mater Sci Mater Med. 2011;22(7):1671-1679.[30]Kuo MC,Yen SK.The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature.Mat Sci Eng C-Mater.2002;20(1-2):153-160.[31]Gong D,Grimes CA,Oomman K,et al.Titanium oxide nanotube arrays prepared by anodic oxidation.J Mater Res. 2001; 16(12): 3331-3334.[32]Tsuchiya H,Macak JM,Muller L,et al.Hydroxyapatite growth on anodic TiO2 nanotubes. J Biomed Mater Res A. 2006; 77(3):534-554.[33]Feng B,Chu X,Chen J,et al.Hydroxyapatite coating on titanium surface with titania nanotube layer and its bond strength to substrate.J Porous Mat.2009;17(4):453-458.[34]Benea L,Mardare-Danaila E,Mardare M,et al.Preparation of titanium oxide and hydroxyapatite on Ti–6Al–4V alloy surface and electrochemical behaviour in bio-simulated fluid solution. Corros Sci.2014;80(3):331-338.[35]Orimo H. The mechanism of minenralization and the role of alkaline phosphatase in health and disease.J Nippon Med Sch.2010;77(1):4-12.[36]Quarles LD,Yohay DA,Lever LW,et al.Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblasts development. J Bone Miner Res. 1992;7(6):683-692. |
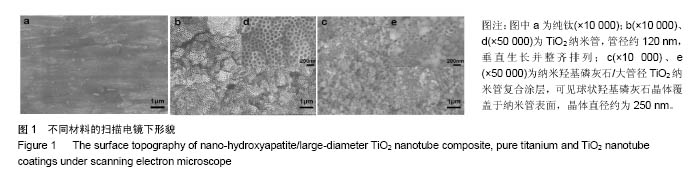
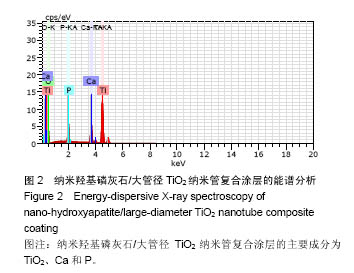
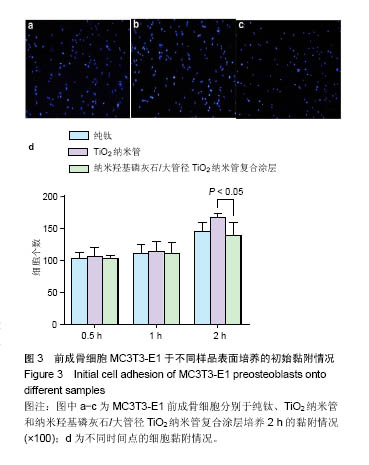
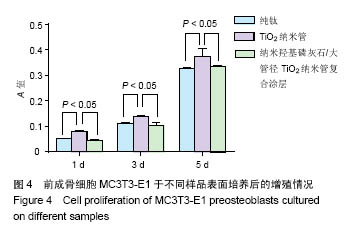
.jpg)
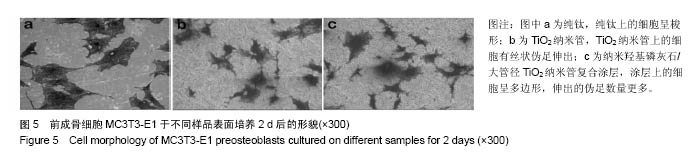
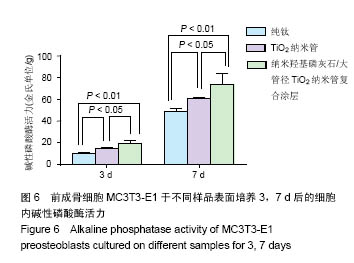
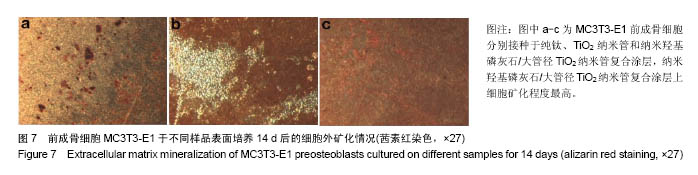
.jpg)