中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (6): 1464-1475.doi: 10.12307/2026.571
• 组织构建综述 tissue construction review • 上一篇 下一篇
骨骼肌衰老主要生理变化及运动的多机制调控作用
侯超文1,2,李兆进2,孔健达2,张树立1
- 1齐鲁理工学院,山东省济南市 250200;2曲阜师范大学体育科学学院,山东省济宁市 272000
-
收稿日期:2024-12-26接受日期:2025-03-06出版日期:2026-02-28发布日期:2025-07-16 -
通讯作者:李兆进,博士,教授,曲阜师范大学体育科学学院,山东省济宁市 272000 共同通讯作者:孔健达,硕士,曲阜师范大学体育科学学院,山东省济宁市 272000 -
作者简介:侯超文,男,1995年生,山东省烟台市人,汉族,2020年曲阜师范大学毕业,硕士,讲师,主要从事体育教学管理和运动康复方面研究工作。 -
基金资助:国家社科规划一般项目(21BTY1115),项目负责人:李兆进;齐鲁理工学院科研计划项目(QIT23SN009),项目负责人:侯超文
Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise
Hou Chaowen1, 2, Li Zhaojin2, Kong Jianda2, Zhang Shuli1
- 1Qilu Institute of Technology, Jinan 250200, Shandong Province, China; 2College of Physical Education, Qufu Normal University, Jining 272000, Shandong Province, China
-
Received:2024-12-26Accepted:2025-03-06Online:2026-02-28Published:2025-07-16 -
Contact:Li Zhaojin, PhD, Professor, College of Physical Education, Qufu Normal University, Jining 272000, Shandong Province, China Co-corresponding author: Kong Jianda, MS, College of Physical Education, Qufu Normal University, Jining 272000, Shandong Province, China -
About author:Hou Chaowen, MS, Lecturer, Qilu Institute of Technology, Jinan 250200, Shandong Province, China; College of Physical Education, Qufu Normal University, Jining 272000, Shandong Province, China -
Supported by:National Social Science Foundation General Project, No. 21BTY1115 (to LZJ); Research Program of Qilu Institute of Technology, No. QIT23SN009 (to HCW)
摘要:
文题释义:
骨骼肌衰老:指随着年龄增长,骨骼肌在细胞结构、生理功能等方面出现的渐进性退变过程,表现为肌肉质量减少、力量下降、功能减退以及对损伤的修复能力变弱等。
运动:是指生物通过身体的动作或位移,包括各种有意识的身体活动如跑步、游泳、跳跃等,以及生物体内部器官和细胞层面的活动,以实现适应环境、维持生命健康、增强体能等目的的行为或过程。
背景:骨骼肌衰老与多种慢性疾病相关,运动被认为是延缓骨骼肌衰老进程的重要手段,但运动干预策略的多机制调控仍需深入探究。
目的:通过综述梳理骨骼肌衰老的主要生理变化,以及探讨运动调控骨骼肌衰老的多重机制,为基础研究和临床应用提供理论依据。
方法:通过第一作者检索 Web of Science、PubMed、Embase、中国知网、万方和维普等数据库,以“骨骼肌衰老,运动调控,慢性炎症,线粒体功能障碍,细胞外基质纤维化,脂质介质,卫星细胞” 为中文检索词,以“skeletal muscle aging,sarcopenia,exercise regulation,physical activity,chronic inflammation,inflammaging,mitochondrial dysfunction,extracellular matrix fibrosis,lipid mediators,satellite cells”为英文检索词,检索从各数据库建库至2024年10月的相关文献,包括研究原著、综述等。依据纳入和排除标准筛选文献,纳入95篇文献进行质量评估和数据提取。
结果与结论:①骨骼肌衰老的核心表现为肌肉质量、力量和功能下降,与多种生理变化密切相关。肌肉中蛋白质的合成能力下降及降解速率加快,导致了肌肉的萎缩与功能衰退。此外,卫星细胞功能障碍亦被认为是肌肉再生能力减弱的关键因素。线粒体功能异常是导致肌肉疲劳与能量代谢紊乱的另一重要因素,直接影响骨骼肌的代谢活性和耐力表现。慢性炎症反应及细胞外基质纤维化则进一步加剧了肌肉衰老,此类因素相互作用,共同导致了骨骼肌退化。②运动被广泛认为是延缓骨骼肌衰老的重要手段。运动通过调节免疫系统减轻骨骼肌的慢性低度炎症反应,增加抗炎因子分泌并抑制促炎因子表达,进而减缓炎症对肌肉的损害。运动还能够提高线粒体的生物合成和功能,增强肌肉的能量代谢能力,进而提升耐力和力量。此外,运动还通过调节脂质代谢和脂质递质的合成,减少脂肪积累,缓解由脂肪引起的炎症反应,进一步保护骨骼肌。运动的机械刺激作用还促进了细胞外基质的重塑,减少纤维化的发生,改善肌肉的结构和功能。同时,运动激活卫星细胞,增强骨骼肌的再生能力,尤其是力量训练和高强度间歇训练对卫星细胞的激活作用尤为显著。③未来的研究包括开展多中心的大规模临床试验以评估长期运动干预对骨骼肌衰老的综合作用。通过分析基因组学、代谢组学等数据,探索不同个体在运动干预中的反应差异,能够为个性化运动干预提供更为精准的理论依据。除了运动,营养补充、药物治疗等其他干预手段对骨骼肌衰老的影响同样不可忽视,未来的研究应探索运动与这些干预手段的联合使用,以期达到更为显著的效果。
https://orcid.org/0009-0000-9287-9873(侯超文);https://orcid.org/0009-0000-0977-801X(李兆进);https://orcid.org/0000-0003-1783-918X(孔健达)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
侯超文, 李兆进, 孔健达, 张树立. 骨骼肌衰老主要生理变化及运动的多机制调控作用[J]. 中国组织工程研究, 2026, 30(6): 1464-1475.
Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475.
随着年龄的增长,尤其是在60岁及以上的群体中,骨骼肌质量显著下降,导致运动能力减弱[12]。骨骼肌衰老在全球范围内普遍存在,特别是在≥65岁的老年群体中更为常见[10]。随着全球人口老龄化趋势的加剧,骨骼肌衰老的患病率预计将进一步上升,成为公共健康领域亟待解决的问题[12]。骨骼肌衰老主要表现为骨骼肌的质量和功能性逐步下降,病理生理特点包括肌纤维数量和体积减少,尤其是快速收缩纤维显著减少[12]。随着衰老,运动能力逐渐减弱,老年人群体尤其受到影响。骨骼肌衰老不仅导致运动能力的丧失,还增加了跌倒、骨折以及长期健康问题的风险[10]。全球范围内,随着老年人口比例的增加,骨骼肌衰老的发病率呈上升趋势,给社会和家庭带来巨大的健康负担[12]。
骨骼肌衰老的机制复杂,涉及多种生物学过程和分子通路,主要机制包括肌肉蛋白质合成与分解失衡、卫星细胞功能衰退、线粒体功能下降、慢性炎症反应以及细胞外基质纤维化等[5,10,13]。在衰老过程中,肌肉的蛋白质合成能力显著下降,导致肌肉质量减少。同时,肌肉蛋白质分解加速,尤其是在低度慢性炎症状态下,肌肉蛋白质分解与肌肉衰退紧密相关。卫星细胞是骨骼肌修复和再生的关键细胞类型。随着衰老,卫星细胞的增殖、分化及修复能力显著减弱,从而导致骨骼肌修复能力下降[14]。线粒体功能下降导致骨骼肌细胞能量供应不足,同时增加氧化应激,进一步加速肌肉细胞损伤和衰老[12]。慢性低度炎症反应是衰老过程中普遍存在的现象。研究发现,衰老引起的慢性炎症反应不仅促进了骨骼肌蛋白质的分解,还加速了骨骼肌的功能衰退[5]。此外,随着衰老,细胞外基质逐渐出现纤维化现象,肌肉的弹性和修复能力受到限制,进一步加剧了衰老过程[10]。
骨骼肌衰老的临床表现主要包括骨骼肌质量和力量下降、肌肉疲劳感增加、运动耐力减退、协调性和灵活性减弱[10,15],这些变化不仅严重影响个体的运动能力,还可能使日常生活活动变得更加困难,并且增加了跌倒及骨折的风险[15]。此外,骨骼肌衰老还与多种慢性疾病的发生密切相关,尤其是代谢综合征、糖尿病、心血管疾病等[2,10]。骨骼肌衰老的加剧不仅影响了个体的生理健康,还显著降低了生活质量[15]。骨骼肌衰老及运动调控骨骼肌衰老的研究时间脉络见表1。
2.2 骨骼肌衰老的主要生理变化 骨骼肌衰老过程中,肌肉组织经历了多种生理变化,这些生理变化相互交织,共同导致肌肉功能的衰退。此部分详细探讨了骨骼肌衰老的主要生理变化,包括慢性炎症、线粒体功能障碍与氧化应激、细胞外基质纤维化、脂质递质参与炎症反应以及卫星细胞调控等方面。骨骼肌衰老的主要生理变化机制见图3,表2。
2.2.1 骨骼肌衰老中的慢性炎症 慢性低度炎症,通常被称为“炎症衰老”,在骨骼肌衰老过程中发挥着关键作用。随着衰老的进程,体内促炎细胞因子如白细胞介素6、肿瘤坏死因子α和C-反应蛋白,在无显著感
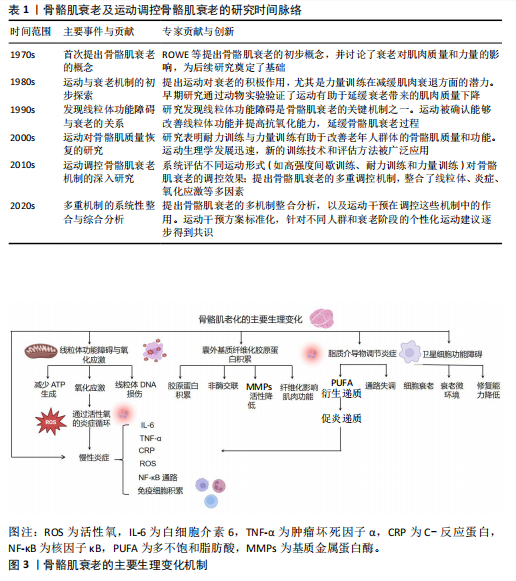

染的情况下持续升高[5-6],这种低度慢性炎症状态不仅促进了肌肉蛋白质的降解,还干扰了肌肉细胞的正常代谢与功能,从而加速骨骼肌质量和力量的下降。
近年来的研究显示,慢性炎症在骨骼肌衰老中的作用不仅限于单纯的炎症因子升高,更涉及多种复杂的分子和细胞机制。首先,促炎细胞因子如白细胞介素6和肿瘤坏死因子α通过激活核因子κB信号通路,促进了肌肉蛋白质合成的抑制与降解的加速[6,16]。核因子κB作为主要的炎症转录因子,调控多种与免疫反应和细胞死亡相关的基因表达。研究表明,核因子κB持续激活不仅抑制了肌肉蛋白质合成,还通过上调自噬途径中的关键蛋白,如Atg7,促进肌肉组织的衰退[16]。
慢性炎症与氧化应激在骨骼肌衰老中的作用尤为突出。随着年龄的增长,炎症引起的活性氧产生显著增加。活性氧不仅作为信号分子调节细胞应答,而且直接损伤细胞成分,包括脂质、蛋白质和DNA,进一步加剧肌肉细胞功能障碍[6]。活性氧增加不仅促进了核因子κB的激活,还加剧了线粒体损伤,形成恶性循环,推动了肌肉的衰老和功能丧失[6]。此外,巨噬细胞在骨骼肌中的积累是骨骼肌衰老的一个重要特征。随着衰老的加剧,巨噬细胞在损伤肌肉中的比例增加,且这些免疫细胞会通过分泌大量的促炎因子如白细胞介素6、肿瘤坏死因子α以及基质金属蛋白酶,进一步加剧局部和系统性炎症反应[17]。这些促炎因子的释放不仅促进肌肉损伤,还抑制肌肉修复,进一步影响骨骼肌的功能恢复。
慢性炎症对骨骼肌修复能力的抑制作用尤为显著。炎症因子通过多种机制抑制骨骼肌卫星细胞(肌肉干细胞)的功能,显著减弱细胞增殖、分化和修复能力[6]。此外,慢性炎症还通过调节细胞因子的水平,改变肌肉前体细胞的微环境,影响其正常功能[6]。针对这些炎症相关的病理机制,近年来的研究发现,ω-3多不饱和脂肪酸[如二十碳五烯酸(eicosapentaenoic acid,EPA)和二十二碳六烯酸(docosahexaenoic acid,DHA)]具有显著的抗炎作用。这些脂肪酸通过抑制白细胞介素6、肿瘤坏死因子α和C-反应蛋白等促炎因子的产生,不仅能减缓衰老过程中慢性炎症的进程,还在缓解衰老相关肌少症方面展现出潜力[6]。ω-3脂肪酸通过减少活性氧的生成,降低氧化应激,进而保护骨骼肌免受损伤。
综上所述,慢性炎症在骨骼肌衰老过程中发挥着多方面且复杂的作用。炎症因子的持续升高通过多种信号通路和分子机制,推进了骨骼肌功能的退化。深入理解慢性炎症的具体机制,尤其是炎症与氧化应激的交互作用,对于开发有效的干预策略,以延缓骨骼肌衰老及相关疾病具有重要意义。
2.2.2 骨骼肌衰老中的线粒体功能障碍和氧化应激 线粒体作为细胞的能量工厂,承担着产生三磷酸腺苷(adenosine triphosphate,ATP)以满足细胞各种能量需求的关键任务[12]。在骨骼肌衰老的过程中,线粒体功能经历了显著的变化,首先表现为线粒体数量减少,这直接影响了细胞的能量供应能力[4];此外,线粒体的形态亦发生了异常变化,如线粒体体积增大和形状圆形化,此类结构变化进一步削弱了线粒体的功能[14];同时,线粒体电子传递链中酶的活性亦出现了显著下降,直接造成了ATP生成效率的降低[12]。这类线粒体功能的改变造成能量代谢紊乱,进而降低了肌肉细胞的功能[14]。氧化应激是线粒体功能障碍的重要后果之一。在正常情况下,线粒体在ATP生成过程中会产生活性氧,活性氧通常通过细胞内的抗氧化系统被有效清除[18]。然而,随着衰老加剧,线粒体功能的持续下降不仅造成活性氧的产生量增加,而且伴随着抗氧化能力的减弱,进而造成细胞内氧化应激水平显著升高[19-20]。过量活性氧不仅直接对肌肉细胞内的蛋白质、脂质和DNA等生物大分子造成损伤,还通过激活多种炎症信号通路,进一步促进骨骼肌的衰老进程[19]。活性氧引起肌肉蛋白质氧化修饰,造成蛋白质结构和功能丧失,这直接影响了骨骼肌的收缩能力和代谢功能[9]。氧化应激还会损伤线粒体DNA,干扰线粒体自噬过程,进而抑制受损线粒体的清除,进一步加剧了线粒体功能障
碍[4,8]。此外,活性氧通过激活核因子κB等促炎信号通路,促进炎症因子的表达,形成了氧化应激与炎症的恶性循环,此机制在骨骼肌衰老中发挥着重要作用[6]。因此,线粒体功能障碍和氧化应激在骨骼肌衰老过程中发挥了重要的作用,成为推动骨骼肌功能下降的关键因素之一。
2.2.3 骨骼肌衰老中的细胞外基质纤维化 细胞外基质在骨骼肌结构和功能中起着至关重要的作用,不仅提供机械支撑、调节细胞间通信,还参与骨骼肌的修复与再生[13,21-22]。细胞外基质主要由胶原蛋白、糖蛋白和蛋白聚糖等大分子组成,形成一个复杂的三维网络结构[23]。然而,随着骨骼肌的衰老加剧,细胞外基质发生了显著变化,表现为纤维化表型的逐步形成[21,23]。
细胞外基质向纤维化表型转变的主要表现包括胶原蛋白积累、非酶交联增加以及基质金属蛋白酶活性下降[24]。随着衰老加剧,骨骼肌内的胶原蛋白周转率降低,造成胶原蛋白在细胞外基质中的积累增加,进而提高肌肉组织的刚性和硬度[21,24],此过程不仅改变了细胞外基质的物理特性,还对骨骼肌功能产生了深远的影响。此外,晚期糖基化终产物等非酶交联物质在肌肉组织中的积累,造成胶原蛋白分子间交联增加,降低了细胞外基质的弹性和可塑性[24],使得细胞外基质更加僵硬,难以适应骨骼肌的动态变化,进一步加剧了纤维化的进程。
另一方面,基质金属蛋白酶如基质金属蛋白酶2和基质金属蛋白酶9在细胞外基质降解中起关键作用[25]。然而,在骨骼肌衰老过程中,基质金属蛋白酶活性显著下降,抑制了细胞外基质的正常重塑过程[21,24],不仅阻碍了纤维化组织的降解,还促使纤维化程度进一步加深,形成恶性循环。细胞外基质纤维化对骨骼肌功能的影响主要体现在以下几个方面:①纤维化使得肌肉组织变得更加僵硬,限制了骨骼肌的伸展和收缩能力,造成运动功能下降[26];②纤维化过程导致基底层增厚,阻止卫星细胞的迁移和分化,影响骨骼肌的再生和修复能力[27];③细胞外基质纤维化还可能影响肌肉组织中的血管生成和神经支配,进一步削弱了骨骼肌的功能和恢复能力[26-27]。
综上所述,细胞外基质向纤维化表型的转变是骨骼肌衰老过程中一个重要病理变化,不仅显著影响了骨骼肌的结构和功能,还显著降低了骨骼肌的修复和再生能力。因此,深入理解细胞外基质纤维化的机制,对于开发有效的抗衰老疗法具有重要意义。
2.2.4 骨骼肌衰老中的脂质递质调控炎症反应 在骨骼肌衰老过程中,脂质递质的动态变化与炎症反应密切相关[28]。脂质递质主要来源于多不饱和脂肪酸,通过酶促反应生成多种具有生物活性的分子,如前列腺素、白三烯、脂氧素、消退素和保护素等[29-30]。因此,这些脂质递质不仅在炎症启动和维持过程中发挥关键作用,还在炎症消退和组织修复中发挥重要功能。
在骨骼肌衰老过程中,脂质递质的作用尤为显著,主要表现在促炎脂质递质的积累、抗炎和促消退脂质递质的不足,以及脂质递质信号通路的失调[31]。随着衰老的进展,促炎脂质递质如前列腺素和白三烯水平持续升高,促进了慢性低度炎症的发生与维持,进而加剧了骨骼肌损伤和功能衰退[32]。同时,衰老可以导致促炎症消退递质生成显著减少,直接造成了炎症消退过程的受阻,使得炎症反应无法有效消退并持续存在[32-33]。此外,脂质递质通过多条信号通路,如5-脂氧合酶、15-脂氧合酶和细胞色素 P450通路调控炎症反应,但在衰老过程中此类通路功能出现失调,造成炎症反应的异常调控,进一步加剧了骨骼肌功能的衰退[31]。因此,脂质递质失调不仅影响了炎症的启动和维持,还使得骨骼肌修复和重塑能力显著下降。
脂质递质在骨骼肌衰老中的作用主要包括调节免疫细胞功能、促进组织修复与再生以及抑制氧化应激。研究表明,促炎症消退递质(如脂氧素和消退素)通过调节巨噬细胞的极化状态,促进巨噬细胞向抗炎表型转化,减少促炎细胞因子的分泌,进而有效促使炎症的消退[34-35]。此外,促炎症消退递质还通过改善成纤维细胞和卫星细胞的功能,推动肌肉组织的修复和再生,进而减缓骨骼肌衰老的进程[34,36]。此外,某些脂质递质具有显著的抗氧化作用,能够减少活性氧的产生和积累,进而保护肌肉细胞免受氧化损伤的侵害[36-37]。脂质递质在延缓骨骼肌衰老过程中发挥着重要的调节和保护作用。然而,随着衰老的进展,脂质递质的生成和调控能力显著下降,这使得骨骼肌无法有效应对持续的炎症刺激,最终形成慢性炎症状态,进一步加速了骨骼肌衰老[38-39]。因此,调控脂质递质的生成和功能对于预防和改善骨骼肌衰老具有重要的临床意义。
综上,衰老造成促炎性脂质递质的积累和抗炎性脂质递质的不足,进而形成慢性低度炎症,进一步加剧了骨骼肌的损伤和功能衰退。脂质递质不仅影响免疫细胞的功能,还促进组织修复和抑制氧化应激。然而,在衰老过程中,脂质递质的生成和信号通路功能失调,导致炎症无法有效消退,妨碍了骨骼肌的修复与再生。因此,恢复脂质递质的平衡显得尤为重要,不仅有助于延缓骨骼肌的衰老进程,还能显著改善骨骼肌的功能状态。
2.2.5 骨骼肌衰老中的卫星细胞调控作用 卫星细胞作为骨骼肌的成体干细胞,位于肌纤维的基底膜与肌细胞之间,承担着骨骼肌修复与再生的关键任务。在骨骼肌衰老的过程中,卫星细胞的数量和功能均发生显著变化,这种变化直接造成了骨骼肌修复和再生能力的下降,进一步加速了骨骼肌衰老的进程[40]。随着衰老加剧,肌肉组织中卫星细胞的数量有所减少,尤其是在Ⅱ型肌纤维中此现象更为显著[26]。然而,也有研究未发现显著的卫星细胞数量变化,这说明衰老对卫星细胞数量的影响可能存在个体差异或受其他因素调节[41]。衰老卫星细胞的增殖和分化潜能显著下降,直接影响了骨骼肌的修复和再生能力[26,42],这种变化与胰岛素样生长因子1信号通路失调密切相关。研究表明,过度的胰岛素样生长因子1反应或失调可能通过调节肌肉抑制素抑制卫星细胞的增殖和分化,进而影响骨骼肌修复[42-43]。此外,卫星细胞的生态位环境亦在衰老过程中发生了显著变化。细胞外基质作为卫星细胞生态位的重要组成部分,其纤维化和结构变化限制了卫星细胞的迁移和分化,进一步削弱了骨骼肌的再生能力[26,42]。
研究发现,当衰老的卫星细胞置于年轻的基质环境时,其功能得到了显著恢复,这说明优化的生态位环境对于维持卫星细胞功能具有重要义[26,42]。
骨骼肌衰老相关的Notch、Wnt和转化生长因子β信号通路发生失衡,这种失衡不仅影响了卫星细胞的活性和功能,而且影响了整个骨骼肌的修复和再生过程[44]。卫星细胞功能下降不仅导致骨骼肌修复和再生能力减弱,还与骨骼肌质量和力量减少密切相关,进一步加剧了骨骼肌衰老的表现[45]。
综上所述,通过调控卫星细胞的数量和功能,恢复细胞增殖和分化能力,有望成为预防和治疗骨骼肌衰老的重要策略。未来的研究应进一步探索卫星细胞在不同环境和信号通路下的具体调控机制,以开发出更加有效的干预方法来延缓或逆转骨骼肌衰老进程。
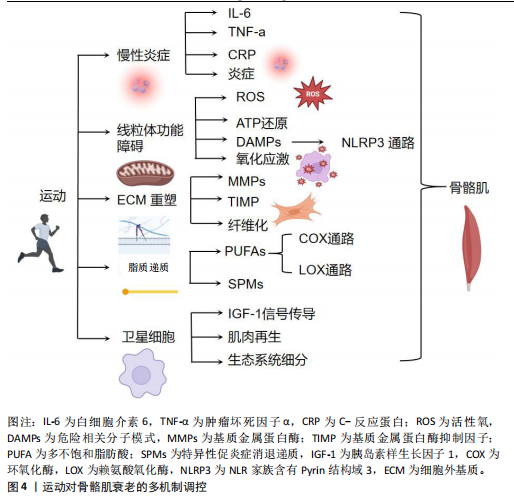
2.3 运动对骨骼肌衰老的多机制调控 衰老使得骨骼肌逐渐经历一系列退行性变化,导致骨骼肌功能和结构受损。运动作为一种关键干预手段,能够通过多种分子和细胞机制减缓骨骼肌衰老,对维持老年人群的肌肉健康和功能具有重要意义。此章节将详细探讨运动如何通过调控慢性炎症、线粒体功能障碍、氧化应激以及细胞外基质重塑等机制,改善骨骼肌衰老。运动对骨骼肌衰老的多机制调控见图4。
2.3.1 运动调控慢性炎症改善骨骼肌衰老 运动在调控衰老相关慢性炎症及改善骨骼肌衰老方面展现出显著的潜力。研究表明,剧烈运动后短暂的急性炎症反应对于骨骼肌修复至关重要,尤其是在受伤后,急性期炎症的及时解决对于长期有益的运动适应和健康组织的维持起着关键作用[46-47]。然而,随着衰老加剧,体内炎症水平往往会增加,即使在无疾病存在的情况下,该现象被称为“炎症衰老”(inflammaging)[48]。衰老通常伴随着促炎蛋白如白细胞介素6、肿瘤坏死因子α和C-反应蛋白水平升高[11],这种慢性低度炎症是多种与年龄相关损伤和慢性疾病的关键因素,包括肥胖、心血管疾病和糖尿病[49],潜在原因包括免疫功能下降、与年龄相关的慢性疾病[50]、氧化应激增加以及抗氧化能力下降[51],这说明随着衰老加剧,体内环境趋向于促进炎症反应。然而,炎症的多因素性质使其起源难以明确界定,尚不清楚是炎症本身引发了衰老相关的病理变化,还是衰老过程造成了炎症水平的升高。此外,基础的低度慢性炎症可能通过引发无法完全解决的过度反应,干扰急性期炎症,进而启动一个有害的循环,造成持续的炎症反应,并最终导致基础免疫谱的永久性改变[52]。然而,抑制急性炎症反应虽然可能减少炎症对组织的损伤,也可能妨碍骨骼肌对运动的有益适应[53],这说明炎症反应在运动后的骨骼肌修复和适应过程中既有利又有弊,需在调控中找到平衡点。
此外,关于使用抗炎药物对运动引起骨骼肌损伤的影响,现有证据尚不明确。有研究指出,在急性炎症的衰老患者中,抗炎药物能够减轻炎症引起的肌肉无力,改善运动表现[54]。然而,缺乏充足的有力证据支持该观点。例如,一项研究发现,衰老在骨骼肌损伤修复和再生方面表现出延迟[55]。此外,衰老在急性期内能够累积更多
的促炎信号,如细胞因子信号抑制因子3、白细胞介素6、肿瘤坏死因子α、白细胞介素8[56],且在运动损伤后的几天内,衰老似乎会阻碍巨噬细胞的有效招募[57]。单核细胞趋化蛋白1在衰老个体中的动力学延迟,造成其峰值出现时间显著滞后[57]。LAVIN等[56]的研究表明,与年轻人相比,老年人的巨噬细胞浸润在24 h和72 h后显著减少。还有研究表明,老年人在运动后4-7 d内巨噬细胞数量持续增加,而年轻人则在此期间恢复至基础水平[58],这说明较高年龄的延长炎症期可能表现为炎症无法有效解决,进而加剧了已存在的全身性炎症。
综上所述,运动在调控衰老相关慢性炎症及改善骨骼肌衰老方面具有重要作用。急性炎症反应在骨骼肌修复和适应过程中不可或缺,但随着衰老加剧,慢性炎症水平升高及其复杂的调控机制对骨骼肌的健康构成了挑战。虽然抗炎药物在一定程度上能够缓解衰老的炎症反应,但长期效果及对运动适应的影响尚需进一步研究。因此,未来研究应重点关注如何在维持急性炎症有益作用的同时,有效抑制慢性炎症的有害影响,进而优化运动干预策略,促进衰老骨骼肌的健康和功能维持。
2.3.2 运动调控线粒体功能障碍和氧化应激改善骨骼肌衰老 衰老能够造成肌肉骨骼中的线粒体功能发生显著变化,这在骨骼肌衰老过程中起到了关键作用。随着衰老的进展,线粒体不仅在形态上表现出增大和圆形化的趋势,数量亦显著减少,酶活性、蛋白质标志物以及线粒体DNA水平同样呈下降趋势[59],这些变化表明线粒体功能障碍可能是导致骨骼肌衰老的重要机制之一。
在衰老过程中,线粒体功能障碍与肌少症密切相关。线粒体受损不仅影响骨骼肌修复过程中的ATP生成,而且限制了修复的程度[60]。此外,线粒体功能下降与肌肉前体细胞的增殖、分化和迁移密切相关[61]。因此,线粒体的健康在维持骨骼肌功能和修复中的作用不可忽视。
运动通过多种机制改善线粒体的功能。运动能够通过增加线粒体的数量和改善线粒体的形态来增强能量代谢功能。例如,耐力运动能促进线粒体生物合成,从而提高肌肉细胞的氧化代谢能力[62-63]。此外,运动还通过激活腺苷单磷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)通路等细胞内信号通路,促进线粒体自噬,帮助清除损伤的线粒体,维护线粒体健康[62],这一过程不仅有助于防止线粒体功能衰退,还能通过减少氧化应激和炎症反应,减缓衰老进程[63]。研究表明,运动可以调节骨骼肌线粒体的稳态,增强抗氧化能力,从而有效预防肌肉衰老。运动通过改善线粒体功能,提高抗氧化能力,从而缓解衰老相关的线粒体功能障碍[8]。此外,过量的线粒体活性氧/活性氮与线粒体能量代谢改变和肌肉衰弱密切相关。活性氧/活性氮的过度产生促进肿瘤坏死因子α释放,并通过激活核因子κB信号通路,引起肌肉蛋白降解的增加,进而加剧肌肉衰老进程[8,12]。
此外,研究表明,运动尤其是长期的耐力训练,可以显著影响系统性炎症因子。耐力训练有助于降低如肿瘤坏死因子α等炎症因子水平,从而减轻由线粒体损伤引起的系统性炎症反应[64-65]。研究指出,长期的耐力训练与肿瘤坏死因子α水平降低相关,这可能有助于缓解肌肉组织修复过程中的炎症反应[64-65]。
总体而言,运动干预通过改善线粒体功能、增强线粒体自噬作用以及调节氧化应激和炎症反应,起到了延缓骨骼肌衰老和促进肌肉修复的关键作用。尽管已有大量研究揭示了运动对线粒体功能的积极影响,仍然需要更多研究来深入探讨不同类型运动对线粒体功能的具体调控机制,特别是在老年群体中的应用。
2.3.3 运动调控细胞外基质向纤维化表型转变改善骨骼肌衰老 骨骼肌的细胞外基质在维持肌肉结构和功能中发挥着关键作用。细胞外基质由胶原成分和大分子(如糖蛋白、蛋白聚糖)构成的三维网络组成,能够提供结构支撑、细胞间通信,并调节肌肉干细胞的功能[66]。细胞外基质由外膜、围肌膜及内膜3层结构组成,内膜进一步细分为基底层和网状层[67]。近年来,研究逐渐认识到细胞外基质在骨骼肌的修复与适应过程中具有动态调控作用。
衰老造成细胞外基质的结构和功能发生显著变化,表现为纤维化表型的逐步显现[68]。随着胶原蛋白周转的减少,肌肉内结缔组织过度积累、非酶交联增加以及胶原蛋白半衰期延长[69],造成骨骼肌硬度增加、力量下降以及受伤风险上升。然而,胶原蛋白的合成与降解之间的平衡对于维持细胞外基质的健康状态至关重要。
在胶原蛋白的降解过程中,基质金属蛋白酶(如基质金属蛋白酶2和基质金属蛋白酶9)起着重要作用,是降解Ⅳ型和Ⅴ型胶原蛋白的主要酶,同时还能降解纤维连接蛋白、蛋白聚糖和层粘连蛋白等大分子[25]。然而,随着衰老加剧,基质金属蛋白酶2和基质金属蛋白酶9对运动引发的细胞外基质降解反应性下降[70],可能是造成胶原蛋白周转和细胞外基质重塑减少的主要原因。此外,基质金属蛋白酶组织抑制因子1/2在衰老动物成纤维细胞中的基因表达增加,进一步抑制了基质金属蛋白酶的活性,促进了细胞外基质的积累和纤维化表型的形成[71]。此外,基底层的逐渐增厚,限制了卫星细胞穿过基底层进行分化和增殖,进而影响了骨骼肌的再生能力[72-73]。运动被认为能够调控细胞外基质向纤维化表型的转变,改善骨骼肌的衰老状态[73-74]。适量的运动刺激能够促进基质金属蛋白酶的活性,增强细胞外基质的动态重塑能力,减少纤维化的积累[74-75]。此外,运动还能够通过调节基质金属蛋白酶组织抑制因子的表达,恢复基质金属蛋白酶与基质金属蛋白酶组织抑制因子之间的平衡,进一步促进细胞外基质的健康状态[72,75]。然而,尚不清楚具体哪种类型和强度的运动最为有效,以及不同个体在响应运动干预时的差异性。因此,未来研究需要重点关注运动干预的最佳方案及在不同年龄和健康状态个体中的效果,以全面理解运动在调控细胞外基质向纤维化表型转变中的作用机制。
综上所述,细胞外基质在骨骼肌衰老过程中经历了显著的结构和功能变化,纤维化表型形成是导致骨骼肌功能下降的重要因素。运动通过调控基质金属蛋白酶和基质金属蛋白酶组织抑制因子的平衡,促进细胞外基质的动态重塑,有望成为改善骨骼肌衰老的有效策略。然而,针对运动干预的具体机制及最佳应用方案仍需进一步深入研究,以期为延缓骨骼肌衰老提供更加科学的依据和方法。
2.3.4 运动通过脂质递质调节炎症反应改善骨骼肌衰老 研究表明,炎症的消退是一个主动的生物过程,而非仅仅依赖于促炎信号的被动减弱[58]。脂质递质在调控局部及全身性炎症反应中发挥着关键作用[76-77]。在基础状态下,源自多不饱和脂肪酸的脂质递质以磷脂的形式存在于细胞膜中,当机体需求时,磷脂酶(如磷脂酶 A2)可迅速释放磷脂,进而转化为活性脂质递质[77]。被动员的多不饱和脂肪酸通过环氧合酶通路、脂氧合酶通路及CYP催化的环氧酶通路等进行氧化,合成了数百种不同的脂质递质[76]。
在组织损伤的早期阶段,促炎脂质递质占据主导地位,典型的炎症表现如发热、发红、疼痛和肿胀均由经典的促炎脂质递质——前列腺素和白三烯驱动[78]。然而,随着炎症的进展,体内脂质递质的谱系发生转变,向抗炎和促消退的方向发展,生成如脂氧素、消退素、保护素等促炎症消退递质[79]。促炎症消退递质作为骨骼肌内的重要信号分子,通过限制多形核白细胞的浸润,并促进巨噬细胞向M2表型极化,进而促进损伤组织的清除和肌肉内稳态的恢复[79-80],这说明促炎症消退递质在炎症消退和骨骼肌修复过程中具有重要的调控作用。
在运动后的恢复期间,外周血液中的脂质递质水平会呈现时序性变化。MARKWORTH等[81]利用代谢脂质组学方法分析了运动后多不饱和脂肪酸代谢产物的变化,在单次高强度运动后,检测到了多种具有抗炎和促消退特性的脂质递质,包括花生四烯酸衍生的脂氧素如脂氧素A4和脂氧素B4、二十碳五烯酸(E系列)和二十二碳六烯酸(D系列)衍生的消退素如消退素 D1和消退素 E1,以及保护素如PD1异构体10S,17S-二羟基二十二碳六烯酸[81],这说明运动后脂质递质的动态变化可能在调节炎症反应和促进骨骼肌修复中发挥重要作用。除MARKWORTH等[81]研究外,还有其他研究表明运动后脂质递质的变化具有时间依赖性。在短期恢复期内,脂质递质的抗炎特性显著增强,有助于调节运动引起的急性炎症反应[82];而在长期恢复期内,这些脂质递质则发挥促进骨骼肌修复和再生的作用。例如,CASTOR-MACIAS等[83]已被证明能够增强肌肉再生,特别是在体积性肌肉损伤后,促进了肌肉组织的修复过程。例如,高强度耐力训练后,二十碳五烯酸(E系列)衍生的消退素水平显著升高,且与肌肉损伤的恢复进程密切相关[84]。此外,在运动后较长时间内,保护素类分子可能在防止肌肉过度损伤和促进长期修复过程中起到关键作用[85]。
然而,在衰老过程中,特异性促消退脂质递质的生成和/或响应似乎存在不足。老龄小鼠在基线状态下即表现出促炎细胞因子、前列腺素和血栓素水平升高,且急性炎症的消退过程显著延迟[77]。在酵母聚糖诱导的腹膜炎模型中,老龄小鼠的消退间隔时间显著延长,并表现出多形核白细胞募集的加剧、单核细胞和巨噬细胞总数的增加以及对凋亡多形核白细胞的摄取受损[86]。此外,特异性促消退脂质递质的时序性水平失调,促消退递质(如RvD1)出现较晚,进一步表明消退阶段存在与年龄相关的损伤[78],这说明在衰老过程中脂质递质调控炎症消退的机制可能发生改变,进而影响骨骼肌的恢复和衰老进程。
综上所述,运动脂质递质在调控炎症反应和改善骨骼肌衰老中具有重要作用。虽然现有研究已揭示了脂质递质在运动后恢复中的时序性变化及在衰老过程中的不足,但尚不清楚不同年龄段个体在脂质递质生成和响应上的具体差异。因此,未来研究需要重点关注脂质递质在衰老相关炎症调控中的具体机制,并探讨通过运动干预优化脂质递质平衡的方法,以期延缓骨骼肌衰老进程,提升老龄人群的骨骼肌健康水平。
2.3.5 运动调控卫星细胞分裂分化改善骨骼肌衰老 卫星细胞作为骨骼肌再生和维持的重要细胞类型受到了广泛关注。卫星细胞是肌纤维再生的基础[87]。在静息状态下,卫星细胞位于肌纤维与基底膜之间,作为骨骼肌修复和再生的储备细胞,维持着骨骼肌的健康和功能。此外,卫星细胞在维持骨骼肌质量方面发挥着重要作用[88]。
运动作为一种有效的刺激因素,能够激活卫星细胞的有丝分裂活动[7],这种高水平的卫星细胞活性会持续存在,直至骨骼肌停止训练[89]。研究表明,卫星细胞的反应能够适应慢性抗阻训练,并在增加肌肉体积中起到主要作用。例如,一项为期16周训练方案的研究显示,卫星细胞的反应比单次力量训练时更为强烈[90],这说明卫星细胞在调节肌纤维大小的长期变化中起着重要作用。然而,衰老能够造成骨骼肌的肥大潜力显著下降,与年轻骨骼肌相比,衰老骨骼肌在相同刺激下不会出现同等程度的肥大[33]。尚不清楚这种差异是源于卫星细胞数量的减少还是卫星细胞增殖潜力的下降,这使得当前研究存在争议。部分研究在小鼠模型中发现卫星细胞数量减少,而另有研究则未发现显著差异[91-92]。此外,研究表明,老年受试者Ⅱ型纤维中的卫星细胞反应显著延迟,但卫星细胞的数量却相近,这说明卫星细胞生态位的变化能够影响骨骼肌再生反应[92]。此外,胰岛素样生长因子1家族被认为是影响衰老卫星细胞功能的关键因素。胰岛素样生长因子1反应的过度和失调与衰老骨骼肌中卫星细胞表达的减少和延迟密切相关[93] 。胰岛素样生长因子1家族可能通过调节肌肉抑制素并抑制卫星细胞的增殖,进而对衰老的卫星细胞产生负面影响[94],这说明胰岛素样生长因子1信号通路失调是造成衰老骨骼肌卫星细胞功能障碍的重要机制之一。卫星细胞的生态位变化仍被认为是造成骨骼肌再生潜力下降的一个重要因素。研究表明,当卫星细胞种植在具有典型年轻特征的基质上时,其功能能够完全恢复[95]。细胞外基质作为卫星细胞生态位的一个重要组成部分,对卫星细胞的功能有显著影响[13]。因此,改善卫星细胞生态位,特别是修复和优化细胞外基质环境,有望恢复衰老骨骼肌的再生能力。
综上所述,运动通过激活卫星细胞的增殖和功能,显著改善骨骼肌的再生能力和质量,延缓骨骼肌衰老。然而,随着衰老加剧,卫星细胞的功能受到多方面因素的影响,包括胰岛素样生长因子1信号通路的失调和生态位的变化,造成骨骼肌肥大潜力下降。虽然现有研究揭示了部分机制,尚不清楚所有影响因素的具体作用路径。因此,未来研究需要重点关注如何通过干预卫星细胞生态位和调控胰岛素样生长因子1等关键信号通路,以进一步提升衰老骨骼肌的再生能力和功能,为抗衰老治疗提供新的策略。

| [1] CRESCIOLI C. Targeting Age-Dependent Functional and Metabolic Decline of Human Skeletal Muscle: The Geroprotective Role of Exercise, Myokine IL-6, and Vitamin D. Int J Mol Sci. 2020;21(3):1010. [2] CARAPETO PV, AGUAYO-MAZZUCATO C. Effects of exercise on cellular and tissue aging. Aging (Albany NY). 2021;13(10): 14522-14543. [3] 李代萍,黄宁,葛美玲,等.中国骨骼肌衰老标志物专家共识(2024)计划书[J].中国循证医学杂志,2024,24(10):1137-1140. [4] 郭辉,孔健达,田春兰.线粒体自噬相关受体蛋白和信号通路在运动防治肌少症中的作用[J].中国组织工程研究,2024, 28(27):4397-4404. [5] TYLUTKA A, WALAS Ł, ZEMBRON-LACNY A. Level of IL-6, TNF, and IL-1β and age-related diseases: a systematic review and meta-analysis. Front Immunol. 2024;15:1330386. [6] CHEN M, WANG Y, DENG S, et al. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front Cell Dev Biol. 2022;10:964130. [7] 邓启烈,莫伟彬.骨骼肌卫星细胞在组织工程与运动损伤修复中的应用[J].中国老年学杂志,2014,34(10):2899-2901. [8] LI J, WANG Z, LI C, et al. Impact of Exercise and Aging on Mitochondrial Homeostasis in Skeletal Muscle: Roles of ROS and Epigenetics. Cells. 2022;11(13):2086. [9] GOMES MJ, MARTINEZ PF, PAGAN LU, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8(12):20428-20440. [10] GUSTAFSSON T, ULFHAKE B. Aging Skeletal Muscles: What Are the Mechanisms of Age-Related Loss of Strength and Muscle Mass, and Can We Impede Its Development and Progression? Int J Mol Sci. 2024;25(20):10932. [11] 徐唯.炎性衰老、肌肉丢失与抗阻运动[J].中国老年学杂志,2016,36(23):6035-6037. [12] BUGLIO AL, BELLANTI F, VENDEMIALE G. The aging muscle: sarcopenia, mitochondrial function, and redox biology. J Gerontol Biol. 2024;72:1-10. [13] 孔健达,穆玉晶,朱磊,等.骨骼肌再生过程中卫星细胞调控机制及其生态位信号的作用[J].中国组织工程研究,2024, 28(7):1105-1111. [14] LEDUC-GAUDET JP, HUSSAIN SNA, BARREIRO E, et al. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int J Mol Sci. 2021;22(15):8179. [15] AGOSTINI D, GERVASI M, FERRINI F, et al. An Integrated Approach to Skeletal Muscle Health in Aging. Nutrients. 2023;15(8):1802. [16] EL ASSAR M, ÁLVAREZ-BUSTOS A, SOSA P, et al. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int J Mol Sci. 2022;23(15):8713. [17] ANTUÑA E, CACHÁN-VEGA C, BERMEJO-MILLO JC, et al. Inflammaging: Implications in Sarcopenia. Int J Mol Sci. 2022;23(23): 15039. [18] ESPINOSA A, CASAS M, JAIMOVICH E. Energy (and Reactive Oxygen Species Generation) Saving Distribution of Mitochondria for the Activation of ATP Production in Skeletal Muscle. Antioxidants (Basel). 2023;12(8):1624. [19] MALDONADO E, MORALES-PISON S, URBINA F, et al. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants (Basel). 2023; 12(3):651. [20] YANG J, LUO J, TIAN X, et al. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants (Basel). 2024;13(4):394. [21] CHEN WJ, LIN IH, LEE CW, et al. Aged Skeletal Muscle Retains the Ability to Remodel Extracellular Matrix for Degradation of Collagen Deposition after Muscle Injury. Int J Mol Sci. 2021; 22(4):2123. [22] GILLIES AR, LIEBER RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318-331. [23] GUMPENBERGER M, WESSNER B, GRAF A, et al. Remodeling the Skeletal Muscle Extracellular Matrix in Older Age-Effects of Acute Exercise Stimuli on Gene Expression. Int J Mol Sci. 2020;21(19):7089. [24] LLOYD SM, HE Y. Exploring Extracellular Matrix Crosslinking as a Therapeutic Approach to Fibrosis. Cells. 2024;13(5):438. [25] 欧阳谭亮,武志娟,钟金城.MMP-2和MMP-9在骨骼肌组织中的研究进展[J].生命科学,2021,33(10):1286-1295. [26] CARECCIA G, MANGIAVINI L, CIRILLO F. Regulation of Satellite Cells Functions during Skeletal Muscle Regeneration: A Critical Step in Physiological and Pathological Conditions. Int J Mol Sci. 2023;25(1):512. [27] SCHÜLER SC, LIU Y, DUMONTIER S, et al. Extracellular matrix: Brick and mortar in the skeletal muscle stem cell niche. Front Cell Dev Biol. 2022;10:1056523. [28] HORNBURG D, WU S, MOQRI M, et al. Dynamic lipidome alterations associated with human health, disease and ageing. Nat Metab. 2023;5(9):1578-1594. [29] NICOLAOU A, MAURO C, URQUHART P, et al. Polyunsaturated Fatty Acid-derived lipid mediators and T cell function. Front Immunol. 2014;5:75. [30] ARITA M. Mediator lipidomics in acute inflammation and resolution. J Biochem. 2012;152(4):313-319. [31] JANNAS-VELA S, ESPINOSA A, CANDIA AA, et al. The Role of Omega-3 Polyunsaturated Fatty Acids and Their Lipid Mediators on Skeletal Muscle Regeneration: A Narrative Review. Nutrients. 2023;15(4):871. [32] MARKWORTH JF, BROWN LA, LIM E, et al. Metabolipidomic profiling reveals an age-related deficiency of skeletal muscle pro-resolving mediators that contributes to maladaptive tissue remodeling. Aging Cell. 2021;20(6):e13393. [33] LI DCW, RUDLOFF S, LANGER HT, et al. Age-Associated Differences in Recovery from Exercise-Induced Muscle Damage. Cells. 2024;13(3):255. [34] KUMAR V, YASMEEN N, CHAUDHARY AA, et al. Specialized pro-resolving lipid mediators regulate inflammatory macrophages: A paradigm shift from antibiotics to immunotherapy for mitigating COVID-19 pandemic. Front Mol Biosci. 2023;10:1104577. [35] KIM N, SHIN HY. Deciphering the Potential Role of Specialized Pro-Resolving Mediators in Obesity-Associated Metabolic Disorders. Int J Mol Sci. 2024;25(17):9598. [36] CUI CY, FERRUCCI L, GOROSPE M. Macrophage Involvement in Aging-Associated Skeletal Muscle Regeneration. Cells. 2023;12(9):1214. [37] VIDELA LA, VALENZUELA R, DEL CAMPO A, et al. Omega-3 Lipid Mediators: Modulation of the M1/M2 Macrophage Phenotype and Its Protective Role in Chronic Liver Diseases. Int J Mol Sci. 2023;24(21):15528. [38] BOZELLI JC JR, AZHER S, EPAND RM. Plasmalogens and Chronic Inflammatory Diseases. Front Physiol. 2021;12:730829. [39] PÉREZ-BAOS S, PRIETO-POTIN I, ROMÁN-BLAS JA, et al. Mediators and Patterns of Muscle Loss in Chronic Systemic Inflammation. Front Physiol. 2018;9:409. [40] 解瑛傲,孔健达,陈芸,等.骨骼肌中卫星细胞衰老生物学机制及潜在的应对策略[J].中国组织工程研究,2024,28(25): 4094-4100. [41] PARKER MH. The altered fate of aging satellite cells is determined by signaling and epigenetic changes. Front Genet. 2015;6:59. [42] FORCINA L, MIANO C, SCICCHITANO BM, et al. Signals from the Niche: Insights into the Role of IGF-1 and IL-6 in Modulating Skeletal Muscle Fibrosis. Cells. 2019;8(3):232. [43] AHMAD SS, AHMAD K, LEE EJ, et al. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells. 2020;9(8):1773. [44] ZHANG W, ZHANG S, XU Y, et al. The DNA Methylation Status of Wnt and Tgfβ Signals Is a Key Factor on Functional Regulation of Skeletal Muscle Satellite Cell Development. Front Genet. 2019;10:220. [45] 孔健达,解瑛傲,陈世娟,等.血流限制训练干预老年肌少症:生物学机制和应用方案建议[J].中国组织工程研究,2024, 28(23):3743-3750. [46] FRANCESCHI C, CAMPISI J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 Suppl 1:S4-9. [47] ZHOU J, ZHANG C, FANG X, et al. Activation of autophagy inhibits the activation of NLRP3 inflammasome and alleviates sevoflurane-induced cognitive dysfunction in elderly rats. BMC Neurosci. 2023;24(1):9. [48] FRANCESCHI C, GARAGNANI P, PARINI P, et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576-590. [49] COWIE CC, RUST KF, BYRD-HOLT DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006; 29(6):1263-1268. [50] CHUNG HY, CESARI M, ANTON S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18-30. [51] KREGEL KC, ZHANG HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292(1): R18-36. [52] WOODS JA, WILUND KR, MARTIN SA, et al. Exercise, inflammation and aging. Aging Dis. 2012;3(1):130-140. [53] PEAKE JM, NEUBAUER O, DELLA GATTA PA, et al. Muscle damage and inflammation during recovery from exercise. J Appl Physiol (1985). 2017;122(3):559-570. [54] ALTURKI M, BEYER I, METS T, et al. Impact of drugs with anti-inflammatory effects on skeletal muscle and inflammation: A systematic literature review. Exp Gerontol. 2018;114:33-49. [55] PEAKE J, DELLA GATTA P, CAMERON-SMITH D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2010; 298(6):R1485-1495. [56] LAVIN KM, PERKINS RK, JEMIOLO B, et al. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985). 2020;128(1):87-99. [57] SORENSEN JR, SKOUSEN C, HOLLAND A, et al. Acute extracellular matrix, inflammatory and MAPK response to lengthening contractions in elderly human skeletal muscle. Exp Gerontol. 2018;106:28-38. [58] Buckley CD, Gilroy DW, Serhan CN, et al. The resolution of inflammation. Nat Rev Immunol. 2013;13(1):59-66. [59] RITOV VB, MENSHIKOVA EV, KELLEY DE. High-performance liquid chromatography-based methods of enzymatic analysis: electron transport chain activity in mitochondria from human skeletal muscle. Anal Biochem. 2004;333(1):27-38. [60] ALWAY SE, PAEZ HG, PITZER CR, et al. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J Cachexia Sarcopenia Muscle. 2023;14(1):493-507. [61] KIM B, KIM JS, YOON Y, et al. Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am J Physiol Regul Integr Comp Physiol. 2013; 305(8):R927-938. [62] GU C, YAN J, ZHAO L, et al. Regulation of Mitochondrial Dynamics by Aerobic Exercise in Cardiovascular Diseases. Front Cardiovasc Med. 2022;8:788505. [63] TREWIN AJ, BERRY BJ, WOJTOVICH AP. Exercise and Mitochondrial Dynamics: Keeping in Shape with ROS and AMPK. Antioxidants (Basel). 2018;7(1):7. [64] WANG YH, TAN J, ZHOU HH, et al. Long-term exercise training and inflammatory biomarkers in healthy subjects: a meta-analysis of randomized controlled trials. Front Psychol. 2023;14:1253329. [65] SELLAMI M, BRAGAZZI NL, ABOGHABA B, et al. The Impact of Acute and Chronic Exercise on Immunoglobulins and Cytokines in Elderly: Insights From a Critical Review of the Literature. Front Immunol. 2021;12:631873. [66] CSAPO R, GUMPENBERGER M, WESSNER B. Skeletal Muscle Extracellular Matrix - What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front Physiol. 2020; 11:253. [67] PURSLOW PP. The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Front Physiol. 2020;11:495. [68] KRAGSTRUP TW, KJAER M, MACKEY AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports. 2011;21(6):749-757. [69] HERCHENHAN A, UHLENBROCK F, ELIASSON P, et al. Lysyl Oxidase Activity Is Required for Ordered Collagen Fibrillogenesis by Tendon Cells. J Biol Chem. 2015;290(26):16440-16450. [70] WESSNER B, LIEBENSTEINER M, NACHBAUER W, et al. Age-specific response of skeletal muscle extracellular matrix to acute resistance exercise: A pilot study. Eur J Sport Sci. 2019;19(3):354-364. [71] STEARNS-REIDER KM, D’AMORE A, BEEZHOLD K, et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell. 2017; 16(3):518-528. [72] NABA A. Mechanisms of assembly and remodelling of the extracellular matrix. Nat Rev Mol Cell Biol. 2024;25(11):865-885. [73] 樊廷俊,田梦,赵君.细胞外基质对细胞行为调控作用的研究进展[J].生命科学, 2021,33(7):844-852. [74] ZHAO P, SUN T, LYU C, et al. Cell mediated ECM-degradation as an emerging tool for anti-fibrotic strategy. Cell Regen. 2023; 12(1):29. [75] REING JE, ZHANG L, MYERS-IRVIN J, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15(3):605-614. [76] 单锴,周姗姗,李茜,等.脂氧合酶在炎症相关疾病中作用的研究进展[J].南京农业大学学报,2025,48(1):27-45. [77] MARKWORTH JF, MADDIPATI KR, CAMERON-SMITH D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc Immunol Rev. 2016; 22:110-134. [78] GIANNAKIS N, SANSBURY BE, PATSALOS A, et al. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat Immunol. 2019;20(5):626-636. [79] SERHAN CN, CHIANG N, DALLI J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27(3): 200-215. [80] CREAN D, GODSON C. Specialised lipid mediators and their targets. Semin Immunol. 2015;27(3):169-176. [81] MARKWORTH JF, VELLA L, LINGARD BS, et al. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1281-1296. [82] MARKWORTH JF, VELLA L, MADDIPATI KR, et al. Inflammation and resolution in exercise-induced skeletal muscle injury: The effect of NSAID treatment on pro-inflammatory and anti-inflammatory/pro-resolving lipid mediators. International Journal of Exercise Science: Conference Proceedings. 2013; 10(1): 34. [83] CASTOR-MACIAS JA, LAROUCHE JA, WALLACE EC, et al. Maresin 1 repletion improves muscle regeneration after volumetric muscle loss. Elife. 2023;12: e86437. [84] RAMOS-CAMPO DJ, ÁVILA-GANDÍA V, LÓPEZ-ROMÁN FJ, et al. Supplementation of Re-Esterified Docosahexaenoic and Eicosapentaenoic Acids Reduce Inflammatory and Muscle Damage Markers after Exercise in Endurance Athletes: A Randomized, Controlled Crossover Trial. Nutrients. 2020;12(3):719. [85] HO ATV, PALLA AR, BLAKE MR, et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc Natl Acad Sci U S A. 2017;114(26): 6675-6684. [86] ARNARDOTTIR HH, DALLI J, COLAS RA, et al. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol. 2014;193(8):4235-4244. [87] BENTZINGER CF, WANG YX, RUDNICKI MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4(2):a008342. [88] PAEZ HG, PITZER CR, ALWAY SE. Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia. Cells. 2023;12(2):249. [89] HAWKE TJ. Expanding Roles for Muscle Satellite Cells in Exercise-Induced Hypertrophy. Function (Oxf). 2020;2(1): zqaa040. [90] NEDERVEEN JP, SNIJDERS T, JOANISSE S, et al. Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R85-R92. [91] BROOKS NE, SCHUENKE MD, HIKIDA RS. No change in skeletal muscle satellite cells in young and aging rat soleus muscle. J Physiol Sci. 2009;59(6):465-471. [92] CONBOY IM, CONBOY MJ, WAGERS AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760-764. [93] MOORE DR, MCKAY BR, TARNOPOLSKY MA, et al. Blunted satellite cell response is associated with dysregulated IGF-1 expression after exercise with age. Eur J Appl Physiol. 2018;118(10):2225-2231. [94] MCKAY BR, OGBORN DI, BAKER JM, et al. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am J Physiol Cell Physiol. 2013;304(8):C717-728. [95] LUKJANENKO L, JUNG MJ, HEGDE N, et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016; 22(8):897-905. |
| [1] | 赵 阳, 李嘉麟, 吴 箫, 邹有瑞, 刘 阳, 马 辉. 胆碱激酶α沉默诱导线粒体功能障碍影响胶质瘤细胞的增殖和凋亡[J]. 中国组织工程研究, 2026, 30(1): 130-138. |
| [2] | 王秋月, 靳 攀, 蒲 锐. 运动干预与细胞焦亡在骨关节炎中的作用[J]. 中国组织工程研究, 2025, 29(8): 1667-1675. |
| [3] | 张晓宇, 韦善文, 方佳炜, 倪 莉. 普鲁士蓝纳米粒子抗氧化恢复退变髓核细胞线粒体功能[J]. 中国组织工程研究, 2025, 29(34): 7318-7325. |
| [4] | 潘 玉, 赵刃峰, 李兴平, 张成栋, 匙 峰, 蒲 超, 罗栩伟, 肖东琴. 铁过载诱导成骨前体细胞铁死亡并抑制成骨分化[J]. 中国组织工程研究, 2025, 29(30): 6381-6390. |
| [5] | 朱梦菡, 杨学涛, 孙一民, 汪成林. 抗炎肽治疗口腔炎症性疾病:调控炎症反应减少组织破坏和结构丧失[J]. 中国组织工程研究, 2025, 29(30): 6529-6537. |
| [6] | 南淞华, 彭超杰, 崔应麟. 线粒体功能障碍与脑衰老:Web of Science核心数据库来源文献的计量学分析[J]. 中国组织工程研究, 2025, 29(26): 5642-5651. |
| [7] | 祝柳慧, 张歆悦, 朱洲海, 杨兴隆, 管 莹, 刘 彬. 卷曲螺旋结构域蛋白2通过促进线粒体自噬抑制帕金森病SH-SY5Y细胞凋亡[J]. 中国组织工程研究, 2025, 29(25): 5403-5413. |
| [8] | 董谷雨, 于 杰, 赵鲁南. 枸杞多糖干预大负荷运动大鼠骨骼肌线粒体和抗氧化酶的效应[J]. 中国组织工程研究, 2025, 29(24): 5134-5139. |
| [9] | 杨 朔, 张 振, 白 硕, 盛 黎, 申 亮, 孙青峰, 高蓓瑶, 葛瑞东, 江 山. 线粒体功能障碍与肌腱病:靶向线粒体治疗的可能性[J]. 中国组织工程研究, 2025, 29(20): 4276-4285. |
| [10] | 樊 饶, 孔健达, 李 琳, 翟 腾, 杨紫柔, 朱 磊. 表观遗传学变化及运动调控:改善骨骼肌衰老的机制[J]. 中国组织工程研究, 2025, 29(2): 419-429. |
| [11] | 李 伟, 尹洪涛, 孙永晨, 徐卫娟, 孙金玲, 金晓东. 线粒体移植治疗肌少症的潜力[J]. 中国组织工程研究, 2025, 29(13): 2842-2848. |
| [12] | 潘世鸿, 刘瑞端. 线粒体自噬与椎间盘退变[J]. 中国组织工程研究, 2024, 28(36): 5872-5876. |
| [13] | 孔健达, 解瑛傲, 马 雯, 刘友涵, 王清路. 帕金森病相关线粒体功能障碍及运动对其潜在的改善作用[J]. 中国组织工程研究, 2024, 28(27): 4413-4420. |
| [14] | 李雁冰, 王记委, 刘晓琴, 郭敏芳, 牛晓洁, 孟 涛, 苏 琴, 王瀚斌, 杨立志, 马存根, 尉杰忠. 灵孢多糖对过氧化氢致SH-SY5Y细胞凋亡及线粒体功能障碍的调控[J]. 中国组织工程研究, 2024, 28(25): 4041-4047. |
| [15] | 葛叡扬, 倪 璨, 杨 琨, 闫福华. 巨噬细胞极化在牙周炎发病及治疗中的作用[J]. 中国组织工程研究, 2024, 28(20): 3246-3251. |
运动被广泛研究用于预防和改善骨骼肌衰老。证据表明,适量的运动不仅能够延缓骨骼肌质量和力量的下降,还能通过改善线粒体功能[4]、调节慢性炎症反应[5-6]、促进卫星细胞活性等多方面发挥抗衰老的作用[7-9]。然而,衰老机体对运动的响应能力可能发生变化,故如何优化运动干预策略以最大化抗衰老效果仍然是当前研究的热点和难点[4,7]。此外,多篇综述探讨了骨骼肌衰老的生理机制及运动干预的作用机制[4,7,9-11],但这些综述仅集中于单一机制的探讨,缺乏对多重调控机制的系统性整合与综合分析。
此综述旨在梳理骨骼肌衰老的主要生理变化,重点探讨运动在调控这些生理变化中的多重机制。通过总结慢性炎症、线粒体功能障碍与氧化应激、细胞外基质纤维化、脂质递质调控炎症反应以及卫星细胞等方面的研究进展,提供一个全面的理论框架,为未来的基础研究和临床应用提供科学依据和指导。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 第一作者于2024年9-10月进行文献检索。
1.1.2 检索文献时限 从各数据库建库至2024年10月发表的相关文献。
1.1.3 检索数据库 主要检索了Web of Science、PubMed、Embase、中国知网、万方和维普等数据库。
1.1.4 检索路径 通过主题词、关键词相结合的方式进行检索,使用布尔逻辑运算符“AND”和“OR”进行连接。
1.1.5 检索文献类型 研究原著、综述、病例报告等。
1.1.6 检索词 以“骨骼肌衰老,运动调控,慢性炎症,线粒体功能障碍,细胞外基质纤维化,脂质介质,卫星细胞” 为中文检索词,以“skeletal muscle aging,sarcopenia,exercise regulation,physical activity,chronic inflammation,inflammaging,mitochondrial dysfunction,extracellular matrix fibrosis,lipid mediators,satellite cells”为英文检索词。
1.1.7 检索策略 以PubMed数据库和CNKI为例,检索策略见图1。
1.1.8 检索文献量 共检索到相关文献2 688篇。
1.2 入选标准
1.2.1 纳入标准 ①研究内容与骨骼肌衰老机制、生理变化或运动调控骨骼肌衰老相关;②研究类型为实验研究、临床研究或高质量综述;③文献语言为英文或英文。
1.2.2 排除标准 ①重复发表的文献;②研究设计不严谨、数据不完整或存在显著错误的文献;③无法获取全文的文献;④学位论文以及会议摘要等。
1.3 文献检索结果 共纳入相关文献95篇,纳入文献流程见图2。
尽管已有大量关于骨骼肌衰老的机制研究,仍然存在一些问题和研究空白:一方面,现有研究多集中于某一特定机制或某一生理过程的独立研究,缺乏对多个机制的系统性整合与分析,未能全面揭示不同机制之间的相互作用与交织影响;另一方面,运动干预对骨骼肌衰老的调控作用仍然存在争议,不同运动形式(如耐力训练、力量训练等)对骨骼肌的影响效果不同,且个体差异(如年龄、性别、运动历史等)亦会显著影响运动效果。此外,对于不同人群对运动的适应性差异,现有文献中缺乏充分的比较和综合分析。
3.2 作者综述区别于他人之篇的特点 此综述的独特之处在于对运动调控骨骼肌衰老的多重机制进行了系统性地整合和综合分析。此文章不仅总结了现有文献中的各个机制(如线粒体功能、慢性炎症、细胞外基质纤维化等)对骨骼肌衰老的影响,还深入探讨了各个机制之间的交互作用,提出运动通过调节多个生理机制共同减缓骨骼肌衰老的观点。此外,此文章还首次将运动对骨骼肌衰老的多重调控作用与不同运动类型的研究成果相结合,突出了运动对骨骼肌衰老的复合效应,尤其是不同运动形式(耐力训练、力量训练、高强度间歇训练等)对骨骼肌衰老的影响。
3.3 综述的局限性 尽管此综述提供了骨骼肌衰老多重机制的综合分析,但仍然存在一些局限性:①关于不同运动类型与骨骼肌衰老的关系,主要集中在文献综述的层面,未能进行深入的实验研究或临床数据分析,导致无法对每种运动形式的具体调节作用进行细致比较和定量分析;②关于不同人群对运动干预的适应性差异,尽管在讨论中提到了此问题,但由于研究的篇幅和主题集中的要求,未能详细展开不同人群对运动的响应差异,尤其是年龄、性别和健康状况对运动效果的影响;③综述中未能涉及骨骼肌衰老与其他器官衰老(如心脏、神经系统等)之间的相互作用,尽管这些相互作用可能对骨骼肌衰老的进程产生重要影响。
3.4 综述的重要意义 尽管存在上述局限性,此综述仍然具有重要的学术价值。通过系统性地整合现有研究成果,此综述提供了骨骼肌衰老的多重机制的全景式视图,强调了运动对骨骼肌衰老的调控作用,尤其是多个生理途径共同作用方面的潜力。通过深入探讨线粒体功能、炎症反应、细胞外基质变化等机制相互作用,为未来研究提供了多维度的思路,强调了多机制干预的重要性。另外,此综述对于运动干预的实践应用具有指导意义。尽管不同运动类型的效果仍存在争议,但此综述揭示了运动对骨骼肌衰老的潜在调控作用,为制定个体化的运动干预策略提供了理论依据,尤其是针对老年人群和长期久坐人群。
3.5 对未来的建议 未来的研究应进一步探索运动对骨骼肌衰老的具体机制,尤其是不同运动形式(如力量训练、耐力训练、高强度间歇训练等)对衰老相关机制的具体调控作用。通过更多的实验和临床研究,可以揭示不同运动方式对线粒体功能、炎症反应、脂质递质动态、细胞外基质变化等机制的具体影响。此外,未来的研究应关注不同个体(如不同年龄段、性别和健康状况人群)对运动的适应性差异。尽管运动对骨骼肌衰老的调节作用已得到初步验证,但如何根据个体差异制定个性化的运动干预策略仍然是一个亟待解决的问题。跨学科的研究将是未来的一个重要方向。通过整合运动生物学、老龄化研究、免疫学等领域的最新成果,研究人员可以更好地理解骨骼肌衰老的多重机制,进一步提升运动干预在延缓骨骼肌衰老过程中的应用价值。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||