中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (13): 2027-2033.doi: 10.12307/2022.328
• 脂肪干细胞 adipose-derived stem cells • 上一篇 下一篇
联合培养血管内皮细胞和脂肪干细胞与部分脱蛋白生物骨构建组织工程骨修复颌骨缺损
廖欣宇,王福科,李彦林,王国梁,杨桂然,侯建飞,杨腾云,钟瑞颖
- 昆明医科大学第一附属医院运动医学科,云南省昆明市 650032
Tissue-engineered bone constructed by co-culture of vascular endothelial cells, adipose derived stem cells, and partially deproteinized biological bone to repair jaw defects
Liao Xinyu, Wang Fuke, Li Yanlin, Wang Guoliang, Yang Guiran, Hou Jianfei, Yang Tengyun, Zhong Ruiying
- Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China
摘要:
文题释义:
部分脱蛋白生物骨:是采用不同理化技术将猪椎骨制备成部分脱蛋白骨后再使用纤维粘连蛋白修饰制成的组织工程骨,具有良好的生物相容性、孔隙率及力学性能,在骨组织工程研究中应用广泛。
血管内皮细胞:参与形成血管内壁的一类细胞,为解决组织工程骨血管化缓慢、新骨生长迟缓等问题,在构建组织工程骨时不仅需要植入有成骨潜能的干细胞,而且同时需植入加速血管形成的内皮细胞。
背景:临界骨缺损的修复一直是困扰临床外科医生的关键问题,骨组织工程研究提供了解决该问题的新思路。如何加快组织工程骨的血管化,进而促进种子细胞的成骨分化是骨组织工程研究中的关键问题。
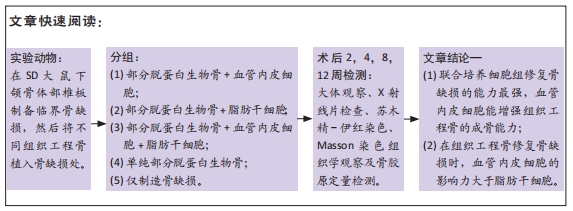
目的:探讨血管内皮细胞和脂肪干细胞联合培养体系与部分脱蛋白生物骨构建组织工程骨修复骨缺损的能力。
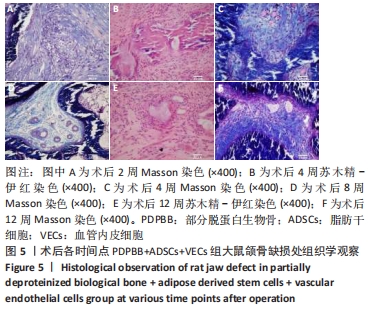
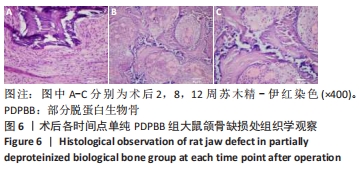
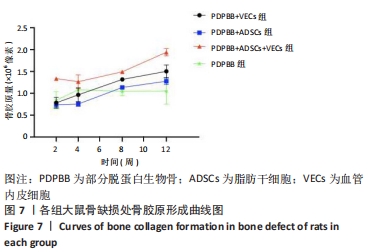
方法:体外构建3种组织工程骨:部分脱蛋白生物骨+血管内皮细胞、部分脱蛋白生物骨+脂肪干细胞、部分脱蛋白生物骨+脂肪干细胞+血管内皮细胞 (血管内皮细胞∶脂肪干细胞比例为 1∶1),以单纯部分脱蛋白生物骨为对照组。取18周龄SD大鼠60只,随机分为5组,每组12只,分别将4种支架材料植入相应的SD大鼠下颌骨骨缺损处,空白对照组仅制造骨缺损,不做修复。术后2,4,8,12周处死动物行大体观察、X射线检查、苏木精-伊红染色和Masson染色组织学观察,并取Masson染色切片行骨胶原定量检测。
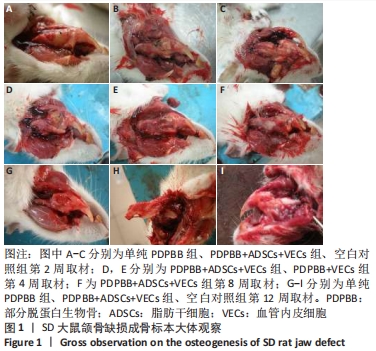
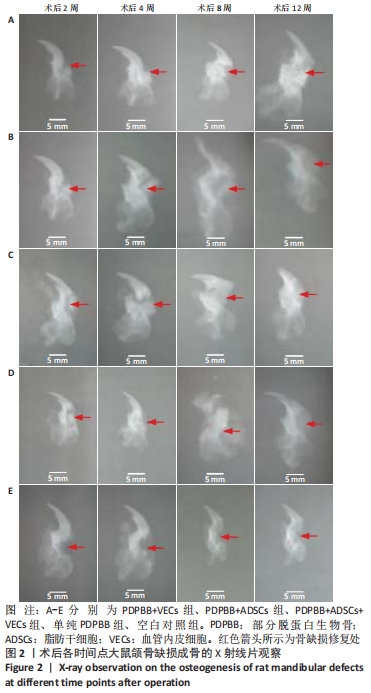
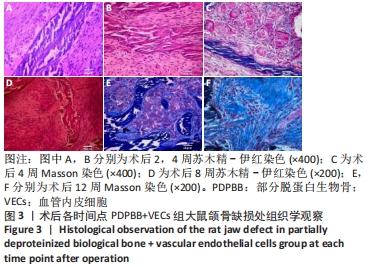
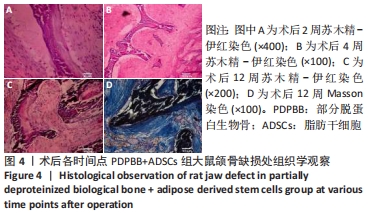
结果与结论:①大体观察示术后部分脱蛋白生物骨+脂肪干细胞+血管内皮细胞组骨支架降解最快,修复骨缺损能力强于其他组,空白对照组骨缺损未被修复;②X射线片示术后8周时部分脱蛋白生物骨+脂肪干细胞+血管内皮细胞组出现骨性联合,骨缝消失,至12周时骨密度明显增加,骨形态已经基本恢复正常;其他组至12周时材料大体形态依然存在,空白对照组骨缺损未被修复;③组织学观察可见部分脱蛋白生物骨+脂肪干细胞组与单纯部分脱蛋白生物骨组比较差异无显著性意义(P=0.607 > 0.05),部分脱蛋白生物骨+血管内皮细胞组与部分脱蛋白生物骨+脂肪干细胞组比较差异有显著性意义(P=0.011 < 0.05),其余各组两两比较差异均有非常显著性意义(P < 0.01);④结果表明,联合培养细胞构建的组织工程骨修复骨缺损能力最强,血管内皮细胞的影响力大于脂肪干细胞。
缩略语:血管内皮细胞:vascular endothelial cells,VECs;脂肪干细胞:adipose-derived stem cells,ADSCs;部分脱蛋白生物骨:partially deproteinised biologic bone,PDPBB
https://orcid.org/0000-0002-8107-7864 (廖欣宇)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号: