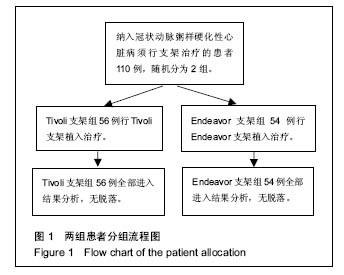
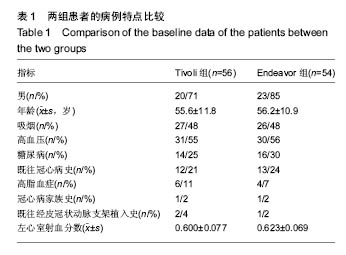
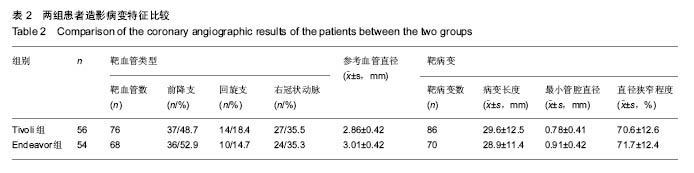
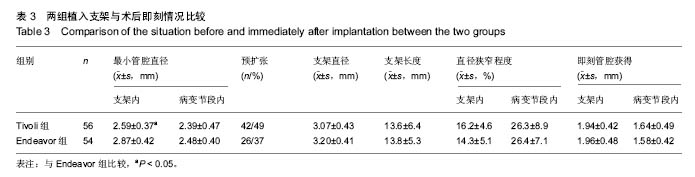
| [1]陈华,赵仙先.生物可降解镁合金支架研究现状[J].介入放射学杂志,2011,20(1):62-64.[2]杨水祥,雷力成,王丽丽,等.新型血管内可吸收镁合金支架的实验研究[J].中华老年心血管病杂志,2012,14(7):743-747.[3]胡晶. 冠状动脉分叉病变单支支架治疗分支是否需要对吻后扩张[J]. 心血管病进展,2014,35(4):458-460.[4]窦克非,徐波,韩亚玲,等.TIVOLI 完全可降解涂层西罗莫司洗脱支架与ENDEAVOR 佐他莫司洗脱支架的临床随访结果[J].中国循环杂志,2012,10(27):334-337. [5]Waksman R ,Pakala R ,Kuchulakanti PK, et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries.Catheter Cardiovasc Interv. 2015;68(4): 607-617.[6]Silber S, AIbertsson P, Avilés FF, et al. Guidelines for percutaneous coronary interventions The Task Force for Percutaneous Coronary Interventions of European Society of Cardiology. Eur Heart J. 2015;26(8):804-847.[7]梁峰,胡大一,方全,等. 2014年ESC/EACTS关于心肌血管重建术的临床指南(三)[J].中国心血管病研究, 2015, 13(3): 212-215.[8]Hillis LD,Smith PK,Andersml JL,et al.2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Hcart Association task force on practice guidelines . developed in collaboration with the American Association for Thoracic Surgery,Society of Cardiovascular Anesthesialogists,and Society ofThoracic Surgeons.J Am Coll Cardiol. 2011;58:e123-210.[9]Han Y,Jing Q ,Xu B,et al. Safety and efficacy of biodegradable polymer-coated sirolimus-eluting stents in “real-world”practice :18-month clinical and 9-month angiographic outcomes. JACC Cardivasc Interv. 2009;2: 303-309.[10]胡大一,施仲伟.心血管疾病防治的新证据、新目标和新策略:美国心脏学院2005年科学大会热点报道[J].中华医学杂志,2005, 85(17):1222-1223.[11]许玉韵. 2005年心血管疾病防治回顾并展望2006年[J].中国医药导刊,2006,8(1):6-9.[12]Lim SS,Gaziano TA,Gakidou E,et al. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries:health effects and costs. Lancet. 2013;370(9604):2054-2062.[13]蒋芳勇. 冠状动脉分叉病变不同介入治疗策略的效果评价[J]. 山东医药,2013,53(22):16-18.[14]周骏,朱海,秦忠. 替罗非班在老年糖尿病合并AMI经皮冠状动脉介入治疗中的价值及安全性评价[J].中西医结合心脑血管病杂志,2016,14(2):158-160.[15]Wang L , Chen CM, Liao WC, et al. Evaluating a community -based stroke nursing education and rehabilitation programme for patients with mild storke. Int J Nurs Pract. 2013;19(3):249-256.[16]Black AT, Clauson M, Fraser S. Nursing education and research rounds:evaluation of a webinar -based education strategy to engage nurses and support practice. J Nurses Prof Dev. 2013;29(5):249-254.[17]郭丽芳.运用护理程序对上消化道出血患者实施健康教育的效果[J].中国实用医刊,2013,40(4):124-125.[18]Forsgren S, Christensen T, Hedemalm A. Evaluation of the case method in nursing education. Nurse Educ Pract. 2014; 14(2):164-169.[19]Tsai HH,Tsai YF,Weng LC, et al. More than communication skills: experiences of communication conflict in nursing home nurses. Med Educ. 2013;47(10):990-1000.[20]胡大一,丁荣晶.2010年调脂治疗领域的争议于进展[J].岭南心血管病杂志,2011,17(4):255-257.[21]窦克非,邱洪,吴元,等.冠心病合并糖尿病患者置入药物洗脱支架和裸金属支架2年临床观察[J].中国循环杂志,2014,25(1):7-10.[22]Hu G,Huang X,Zhang X,et al. Anti-inflammatory effect of B-type natriuretic peptide postconditioning during myocardial ischemia-reperfusion :involvement of PI3K /AKt signaling pathway. Inflammation. 2014;37(5):1669-1674.[23]Stone GW,Teirstein PS,Meredith IT, et al. A prospective,randomized evaluation of a novel everolimus-eluting coronary stent, the PLATINUM (a Prospective, Randomized, Multicenter Trial to Asses an Everolimus-Eluting Coronary Stent System [PROMUS Element ] for the Treatment of Up to Two de Novo Coronary Arrtery Lesions) trial. J Am Coll Cardiol. 2011;57(16): 1700-1708.[24]Urban P,Abizaid A, Banning A,et al. Stent thrombosis and bleeding complications after implantation of sirolimus-eluting coronayt stents in an unselecyed worldwide population, a report from the e-SELECT (Muti-Center Post-Market Surveillance) registry. J Am Coll Cardiol. 2011;57(13):1445-1454.[25]Kimura T,Morimoto T, Nakagawa Y, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent .ENDEAVOR I In vestigators. Four- year cinical follow-up after implantation of the endeavor zotarolimus-eluting stent: ENDEAVOR I ,the first-in-human study. Am J Cardiol. 2007;100(8B):56-61M.[26]Wong N. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276.[27]黄琼,张新风. 高敏CRP与冠脉狭窄程度及支架术后再狭窄相关性[J].中国医药导报,2014,16(18):1197-1199.[28]陈明,陈志武,龙子江.炎性标志物对心血管疾病风险的预测及意义[J]. 心血管病进展,2013,34(2):290-295.[29]于萍,蔡英,周晓茜,等.血清超敏肌钙蛋白T轻度升高在老年冠心病患者中的临床意义[J].临床心血管病杂志, 2012,28(3): 206-208.[30]Lee CW,Suh J,Lee SW,et al.Factors predictive of cardiac events and restenosis after siolimus-eluting stene implantation in small coronary arteries. Catheter Cardiovasc Interv. 2006;10:1002-1019.[31]Nagaya N,Nishikiml T,Goto Y,et al. Plasma brain natriuretic peptide is a biochemical marker for the prediction of progressive vetricular remodeling after acute myocardial infarction. Am Heart J. 1998;135:21-28.[32]重组人脑利钠肽临床研究协作组.重组人脑利钠肽和硝酸甘油治疗急性失代偿心力衰竭疗效和安全性的随机、开放、平行对照的多中心临床研究[J].中华心血管病杂志, 2006,34(3): 222-226.[33]Connel JM, Davies E. The new biology of aldosterone. J Endorinol. 2015;186:1-20. [34]Cowle MR, Mendez GF.BNP and congestive heart failure. Prog Cardiovase Dis. 2014;44:293-321.[35]Kelbaek H,Thuesen L,Helqvist S,et al.The Stenting Coronary Arteries in Non-stress/benestent disease (SCANDSTENT)trial. J Am Coll Cardiol. 2006;47(2):449-455.[36]Lee SW,Park SW, Kim YH,et al.A randomized comparision of sieolimus -versus paclitaxel-eluting stent implantation in patients with diabetes mellitus: 4-year clinical coutcomes of DES-DIABETES(drug-eluting stent in patients with DIABETES mellitus) trial. JACC Cardiovasc Interv. 2011;4(3): 310-316.[37]Kim MC,Jeong MH,Ahn Y, et al. What is optimal revasculaeization strategy in patients with multivessel coronary artery disease in non-ST-elevation myocardial infarction? Multivessel or culprit-only revascularization. J Int Cardiol. 2014;24(2):148-153.[38]中华医学会心血管病分会,中华心血管病杂志编辑委员会.非ST段抬高急性冠脉综合征诊断和治疗指南[J].中华心血管病杂志, 2012,40(5):353-367.[39]蒋芳勇. 冠状动脉分叉病变不同介入治疗策略效果评价[J]. 山东医药,2013,53(22):16-18. |
.jpg)
.jpg)