| [1] Duncan JG. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta. 2011;1813(7): 1351-1359.
[2] Anderson EJ, Kypson AP, Rodriguez E, et al. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54(20): 1891-1898.
[3] Liang Q, Kobayashi S. Mitochondrial quality control in the diabetic heart. J Mol Cell Cardiol. 2016;95:57-69.
[4] Baughman JM, Perocchi F, Girgis HS, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476 (7360):341-345.
[5] Perocchi F, Gohil VM, Girgis HS, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467(7313):291-296.
[6] Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464(7293):1313-1319.
[7] Mallilankaraman K, Doonan P, Cárdenas C, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151(3):630-644.
[8] Wheeler DG, Groth RD, Ma H, et al. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149(5):1112-1124.
[9] Csordás G, Golenár T, Seifert EL, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca²? uniporter. Cell Metab. 2013;17(6): 976-987.
[10] Cheng A, Yang Y, Zhou Y, et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016;23(1):128-142.
[11] Mallilankaraman K, Doonan P, Cárdenas C, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151(3):630-644.
[12] Kevin Foskett J, Madesh M. Regulation of the mitochondrial Ca(2+) uniporter by MICU1 and MICU2. Biochem Biophys Res Commun. 2014;449(4):377-383.
[13] Marin V, Stornaiuolo A, Piovan C, et al. RD-MolPack technology for the constitutive production of self-inactivating lentiviral vectors pseudotyped with the nontoxic RD114-TR envelope. Mol Ther Methods Clin Dev. 2016;3:16033.
[14] De Groote P, Grootjans S, Lippens S, et al. Generation of a new Gateway-compatible inducible lentiviral vector platform allowing easy derivation of co-transduced cells. Biotechniques. 2016;60(5):252-259.
[15] Jang YH, Song HI, Yang Y, et al. Reliable RT-qPCR-based titration of retroviral and lentiviral vectors via quantification of residual vector plasmid DNA in samples. Biotechnol Lett. 2016;38(8):1285-1291.
[16] Kafaie J, Song R, Abrahamyan L, et al. Mapping of nucleocapsid residues important for HIV-1 genomic RNA dimerization and packaging.Virology. 2008; 375(2):592-610.
[17] LaBarre DD, Lowy RJ. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J Virol Methods. 2001;96(2):107-126.
[18] Nauta JJ, de Bruijn IA. On the bias in HI titers and how to reduce it. Vaccine. 2006;24(44-46):6645-6646.
[19] Fan J, Guan S, Cheng CF, et al. PKCdelta clustering at the leading edge and mediating growth factor-enhanced, but not ecm-initiated, dermal fibroblast migration. J Invest Dermatol. 2006;126(6):1233-1243.
[20] Chen M, Li W, Fan J, et al. An efficient gene transduction system for studying gene function in primary human dermal fibroblasts and epidermal keratinocytes. Clin Exp Dermatol. 2003;28(2):193-199.
[21] Kevin Foskett J, Madesh M. Regulation of the mitochondrial Ca(2+) uniporter by MICU1 and MICU2. Biochem Biophys Res Commun. 2014;449(4):377-383.
[22] Aichberger KJ, Mittermann I, Reininger R, et al. Hom s 4, an IgE-reactive autoantigen belonging to a new subfamily of calcium-binding proteins, can induce Th cell type 1-mediated autoreactivity. J Immunol. 2005; 175(2):1286-1294.
[23] de la Fuente S, Matesanz-Isabel J, Fonteriz RI, et al. Dynamics of mitochondrial Ca2+ uptake in MICU1-knockdown cells. Biochem J. 2014;458(1):33-40.
[24] Hoffman NE, Chandramoorthy HC, Shamugapriya S, et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep. 2013;5(6):1576-1588.
[25] Tian Y, Qin L, Qiu H, et al. RPS3 regulates melanoma cell growth and apoptosis by targeting Cyto C/Ca2+/MICU1 dependent mitochondrial signaling. Oncotarget. 2015;6(30):29614-29625.
[26] Antony AN, Paillard M, Moffat C, et al. MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat Commun. 2016;7:10955.
[27] Zhou X, Ren Y, Kong L, et al. Targeting EZH2 regulates tumor growth and apoptosis through modulating mitochondria dependent cell-death pathway in HNSCC. Oncotarget. 2015;6(32):33720-33732.
[28] Mattila M, Koskenvuo J, Söderström M, et al. Intramyocardial injection of SERCA2a-expressing lentivirus improves myocardial function in doxorubicin-induced heart failure. J Gene Med. 2016;18(7):124-133.
[29] Shen SH, Yu N, Xu H, et al. Inhibition of Human Glioma Cell Proliferation Caused by Knockdown of Utrophin Using a Lentivirus-Mediated System. Cancer Biother Radiopharm. 2016;31(4):133-138.
[30] Yue B, Lin Y, Ma X, et al. Survivin-TGFB3-TIMP1 Gene Therapy Via Lentivirus Vector Slows the Course of Intervertebral Disc Degeneration in an In Vivo Rabbit Model. Spine (Phila Pa 1976). 2016;41(11):926-934.
[31] 张磊,刘庆友,胡天,等.第三代慢病毒高效率包装系统的建立[J].基因组学与应用生物学,2009,28(2):326-330. |
.jpg) 文题释义:
线粒体功能紊乱:主要以线粒体钙稳态失衡为角度阐述线粒体功能紊乱在细胞生物学功能中所发挥的作用。细胞线粒体钙主要与细胞胞浆钙稳态、线粒体三羧酸循环关键酶、细胞自噬、生存、死亡以及线粒体的空间位置等关系密切。
线粒体钙离子单向转运复合体:目前研究发现,该复合体主要以构成线粒体内膜孔道的线粒体钙离子单向转运蛋白,以及调剂其活性的MICU1,MICU2,MCUR1及EMRE组成,共同完成细胞线粒体钙摄取的精密调节。
文题释义:
线粒体功能紊乱:主要以线粒体钙稳态失衡为角度阐述线粒体功能紊乱在细胞生物学功能中所发挥的作用。细胞线粒体钙主要与细胞胞浆钙稳态、线粒体三羧酸循环关键酶、细胞自噬、生存、死亡以及线粒体的空间位置等关系密切。
线粒体钙离子单向转运复合体:目前研究发现,该复合体主要以构成线粒体内膜孔道的线粒体钙离子单向转运蛋白,以及调剂其活性的MICU1,MICU2,MCUR1及EMRE组成,共同完成细胞线粒体钙摄取的精密调节。.jpg) 文题释义:
线粒体功能紊乱:主要以线粒体钙稳态失衡为角度阐述线粒体功能紊乱在细胞生物学功能中所发挥的作用。细胞线粒体钙主要与细胞胞浆钙稳态、线粒体三羧酸循环关键酶、细胞自噬、生存、死亡以及线粒体的空间位置等关系密切。
线粒体钙离子单向转运复合体:目前研究发现,该复合体主要以构成线粒体内膜孔道的线粒体钙离子单向转运蛋白,以及调剂其活性的MICU1,MICU2,MCUR1及EMRE组成,共同完成细胞线粒体钙摄取的精密调节。
文题释义:
线粒体功能紊乱:主要以线粒体钙稳态失衡为角度阐述线粒体功能紊乱在细胞生物学功能中所发挥的作用。细胞线粒体钙主要与细胞胞浆钙稳态、线粒体三羧酸循环关键酶、细胞自噬、生存、死亡以及线粒体的空间位置等关系密切。
线粒体钙离子单向转运复合体:目前研究发现,该复合体主要以构成线粒体内膜孔道的线粒体钙离子单向转运蛋白,以及调剂其活性的MICU1,MICU2,MCUR1及EMRE组成,共同完成细胞线粒体钙摄取的精密调节。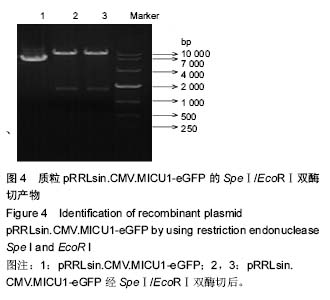

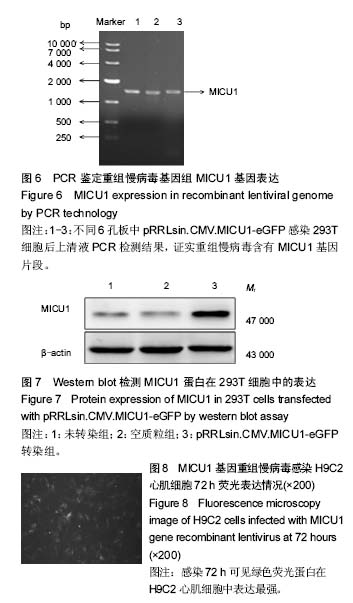
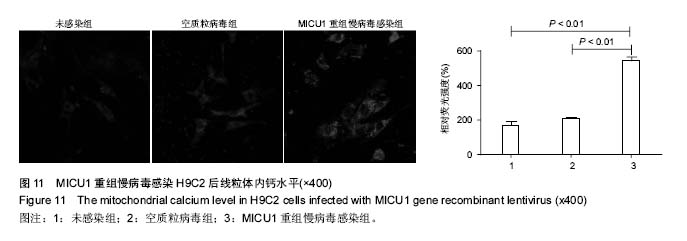
.jpg)
.jpg) 文题释义:
线粒体功能紊乱:主要以线粒体钙稳态失衡为角度阐述线粒体功能紊乱在细胞生物学功能中所发挥的作用。细胞线粒体钙主要与细胞胞浆钙稳态、线粒体三羧酸循环关键酶、细胞自噬、生存、死亡以及线粒体的空间位置等关系密切。
线粒体钙离子单向转运复合体:目前研究发现,该复合体主要以构成线粒体内膜孔道的线粒体钙离子单向转运蛋白,以及调剂其活性的MICU1,MICU2,MCUR1及EMRE组成,共同完成细胞线粒体钙摄取的精密调节。
文题释义:
线粒体功能紊乱:主要以线粒体钙稳态失衡为角度阐述线粒体功能紊乱在细胞生物学功能中所发挥的作用。细胞线粒体钙主要与细胞胞浆钙稳态、线粒体三羧酸循环关键酶、细胞自噬、生存、死亡以及线粒体的空间位置等关系密切。
线粒体钙离子单向转运复合体:目前研究发现,该复合体主要以构成线粒体内膜孔道的线粒体钙离子单向转运蛋白,以及调剂其活性的MICU1,MICU2,MCUR1及EMRE组成,共同完成细胞线粒体钙摄取的精密调节。