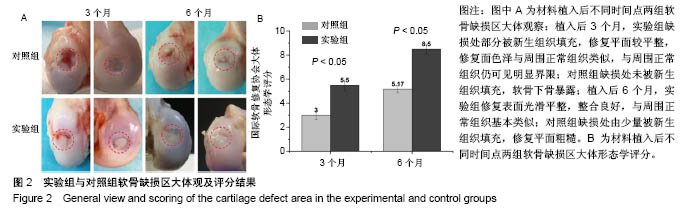
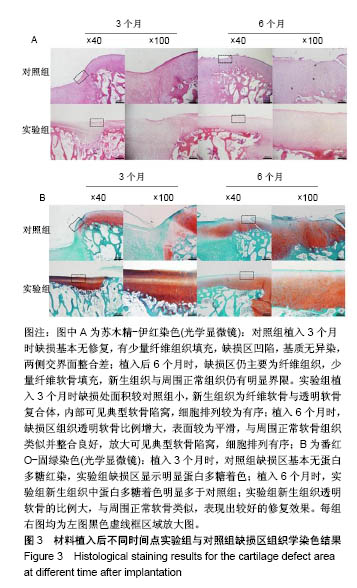
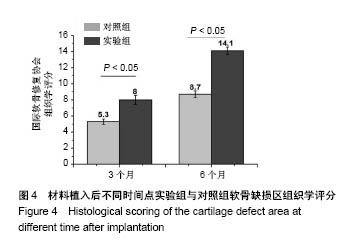
| [1] Lourenco S, Lucas R,Araujo F,et al. Osteoarthritis medical labelling and health-related quality of life in the general population. Health Qual Life Outcomes.2014;12:146.[2] Dell’accio F,Vincent TL.Joint surface defects: clinical course and cellular response in spontaneous and experimental lesions. Eur Cell Mater.2010;20:210-217.[3] Makris EA,Gomoll AH,Malizos KN,et al. Repair and tissue engineering techniques for articular cartilage.Nat Rev Rheumatol. 2015;11:21-34.[4] Huang H,Zhang X,Hu X,et al.A functional biphasic biomaterial homing mesenchymal stem cells for in vivo cartilage regeneration. Biomaterials.2014;35:9608-9619.[5] Ko JY, Im GI.Chondrogenic and Osteogenic Induction from iPS Cells. Methods Mol Biol. 2016;1357:441-450[6] Johnson K,Zhu S,Tremblay MS,et al.A stem cell-based approach to cartilage repair.Science. 2012;336:717-721.[7] Mei L, Shen B, Ling P, et al.Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model.PloS One.2017;12:e0176107.[8] Dubey NK,Mishra VK,Dubey R,et al.Combating Osteoarthritis through Stem Cell Therapies by Rejuvenating Cartilage: A Review.Stem Cells Int.2018;2018:5421019.[9] Jones EA,English A,Henshaw K,et al.Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum.2004;50(3):817-827.[10] van Lent PL,van den Berg WB. Mesenchymal stem cell therapy in osteoarthritis: advanced tissue repair or intervention with smouldering synovial activation? Arthritis Res Ther. 2013; 15(2):112.[11] 卢强,张莉,彭江,等.可注射性组织工程软骨源性微载体的微观结构及其生物相容性观察[J].中国矫形外科杂志, 2009,17(9): 688-691.[12] Sutherland AJ,Converse GL,Hopkins RA,et al.The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv Healthc Mater.2015;4(1):29-39.[13] Olvera D,Daly A,Kelly DJ.Mechanical Testing of Cartilage Constructs. Methods Mol Biol. 2015;1340: 279-287[14] DiSilvestro MR,Suh JK.Biphasic poroviscoelastic characteristics of proteoglycan-depleted articular cartilage: simulation of degeneration. Ann of Biomed Eng. 2002,30: 792-800[15] Yin H,Wang Y,Sun Z,et al.Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles.Acta Biomater. 2016;33:96-109.[16] Hsieh YH,Shen BY,Wang YH,et al.Healing of Osteochondral Defects Implanted with Biomimetic Scaffolds of Poly(epsilon-Caprolactone)/Hydroxyapatite and Glycidyl-Methacrylate-Modified Hyaluronic Acid in a Minipig. Int J Mol Sci.2018;19(4).pii: E1125.doi: 10.3390/ijms19041125.[17] Fisher MB,Belkin NS,Milby AH,et al.Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: implications for tissue engineering.Tissue Eng Part A. 2015;21(3-4):850-860. [18] Doyran B,Tong W,Li Q,et al.Nanoindentation modulus of murine cartilage: a sensitive indicator of the initiation and progression of post-traumatic osteoarthritis.Osteoarthritis Cartilage. 2017;25(1):108-117. [19] Chang NJ,Lin CC,Shie MY, et al.Positive effects of cell-free porous PLGA implants and early loading exercise on hyaline cartilage regeneration in rabbits.Acta Biomater. 2015;28: 128-137.[20] Mainil-Varlet P, Aigner T, Brittberg M, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am.2003;85-A Suppl 2:45-57.[21] Mattei G,Gruca G,Rijnveld N,et al.The nano-epsilon dot method for strain rate viscoelastic characterisation of soft biomaterials by spherical nano-indentation. J Mech Behav Biomed Mater. 2015;50:150-159. [22] Jeznach O, Kolbuk D, Sajkiewicz P. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J Biomed Mater Res A.2018.doi: 10.1002/jbm.a.36449.[Epub ahead of print] Review.[23] 方洪松,周建林,彭昊,等.组织工程支架材料修复关节软骨缺损[J].中国组织工程研究,2016,20(52): 7891-7898[24] Polat G,Ersen A,Erdil ME,et al.Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1299-1303.[25] Nooeaid P,Salih V,Beier JP,et al.Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16(10):2247-2270.[26] Vinatier C,Guicheux J.Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann Phys Rehabil Med.2016;59(3):139-144.[27] Agarwal T,Narayan R,Maji S,et al. Gelatin/Carboxymethyl chitosan based scaffolds for dermal tissue engineering applications. Int J Biol Macromol.2016;93(Pt B):1499-1506.[28] Cheng NC,Estes BT,Awad HA,et al.Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15(2):231-241. [29] 侯立刚,杨建义.骨修复中应用的生物降解可吸收材料[J].中国组织工程研究,2016,20(3):441-445.. [30] Christensen BB,Foldager CB,Olesen ML,et al.Experimental articular cartilage repair in the Gottingen minipig: the influence of multiple defects per knee.J Exp Orthop.2015;2(1):13. [31] Gotterbarm T,Breusch SJ,Schneider U,et al.The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim. 2008;42(1):71-82.[32] Baboolal TG,Mastbergen SC,Jones E,et al.Synovial fluid hyaluronan mediates MSC attachment to cartilage, a potential novel mechanism contributing to cartilage repair in osteoarthritis using knee joint distraction. Ann Rheum Dis. 2016;75(5):908-915.[33] Saarakkala S,Korhonen RK,Laasanen MS,et al. Mechano-acoustic determination of Young’s modulus of articular cartilage. Biorheology.2004;41(3-4):167-179.[34] Bhumiratana S, Eton RE, Oungoulian SR, et al. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A. 2014 ;111(19):6940-6945. |
.jpg)


.jpg)