中国组织工程研究 ›› 2019, Vol. 23 ›› Issue (2): 165-171.doi: 10.3969/j.issn.2095-4344.0692
• 组织工程骨及软骨材料 tissue-engineered bone and cartilage materials • 下一篇
钛铜合金纳米管形貌能够降低细菌活性并促进成骨细胞功能
张 磊1,张杭州2,Allieu Kamara3
- 1锦州医科大学附属第三医院骨一科,辽宁省锦州市 121001;2中国医科大学附属第一医院运动医学与关节外科,辽宁省沈阳市 110001;3中国医科大学,辽宁省沈阳市 110001
-
出版日期:2019-01-18发布日期:2019-01-18 -
通讯作者:Allieu Kamara,中国医科大学,辽宁省沈阳市 110001 -
作者简介:张磊,男,1978年生,辽宁省沈阳市人,汉族,主治医师,主要从事膝关节周围损伤及骨病研究。 -
基金资助:国家自然科学基金(81671811);国家自然科学基金青年科学基金项目(81501857,项目负责人:张杭州);辽宁省省直医院改革重点临床科室诊疗能力建设项目(LNCCC-A03-2014);辽宁省自然基金项目(LK201642);沈阳市人口与健康科技攻关专项项目(F15-139-9-23)
Titanium-copper alloys with nanotubular coatings increase antibacterial abilities and osteoblast functions
Zhang Lei1, Zhang Hangzhou2, Allieu Kamara3
- 1First Department of Orthopedics, Third Affiliated Hospital of Jinzhou Medical University, Jinzhou 121001, Liaoning Province, China; 2Department of Sports Medicine/Joint Surgery, First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China; 3China Medical University, Shenyang 110001, Liaoning Province, China
-
Online:2019-01-18Published:2019-01-18 -
Contact:Allieu Kamara, China Medical University, Shenyang 110001, Liaoning Province, China -
About author:Zhang Lei, Attending physician, First Department of Orthopedics, Third Affiliated Hospital of Jinzhou Medical University, Jinzhou 121001, Liaoning Province, China -
Supported by:the National Natural Science Foundation of China, No. 81671811; the National Natural Science Foundation of China (Youth Foundation), No. 81501857 (to ZHZ); Liaoning Provincial Reform Project for Key Clinical Diagnosis and Treatment, No. LNCCC-A03-2014; the Natural Science Foundation of Liaoning Province, No. LK201642; Special Science and Technology Research Project for Population and Health in Shenyang, No. F15-139-9-23
摘要:
文章快速阅读:
.jpg)
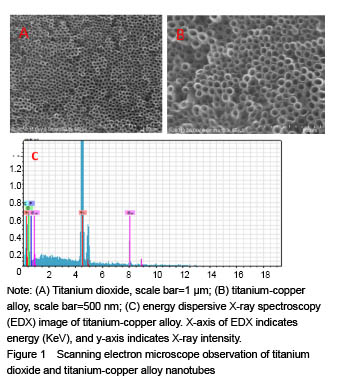
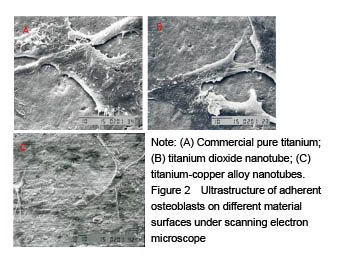
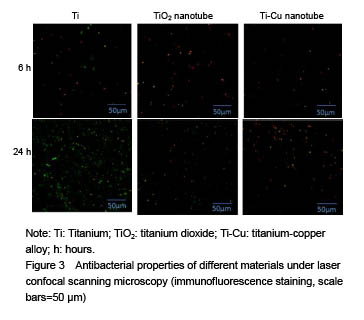
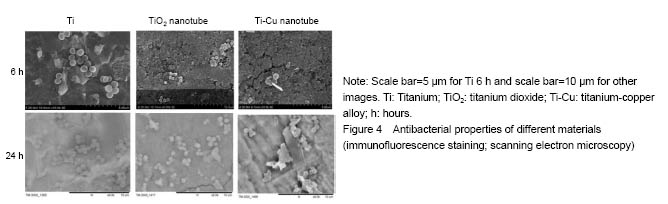
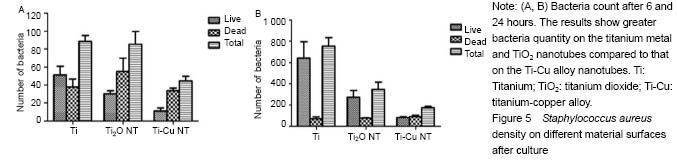
结果与结论:①扫描电镜可见,钛纳米管和钛-铜合金纳米管表面良好的细胞黏附性,小鼠成骨细胞形态良好,排列规则;纳米管组细胞增殖情况优于纯钛组,而钛-铜合金纳米管与钛纳米管组差异无显著性意义;②细菌黏附实验显示,与钛铜合金纳米管相比,钛金属和TiO2纳米管上的细菌数量更多;③结果证实,钛-铜合金纳米管对细菌黏附具有良好的抑制作用,不影响成骨细胞的生物功能。
orcid: 0000-0003-3897-2852(Zhang Lei)
中图分类号:
引用本文
张 磊,张杭州,Allieu Kamara. 钛铜合金纳米管形貌能够降低细菌活性并促进成骨细胞功能[J]. 中国组织工程研究, 2019, 23(2): 165-171.
Zhang Lei, Zhang Hangzhou, Allieu Kamara. Titanium-copper alloys with nanotubular coatings increase antibacterial abilities and osteoblast functions[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 165-171.
Adhesion and proliferation of mouse osteoblasts on different material surfaces
Antibacterial property testing
| [1] Weiss RJ, Thorsell M, Stark A, et al. 2- to 9-year outcome of stemmed total knee arthroplasty. Similar failure rates in patients when used primary or as a revision. Acta Orthop. 2014;85(6):609-613. [2] Dale H, Hallan G, Hallan G, et al. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop. 2009;80(6):639-645. [3] Martínez-Pastor JC, Maculé-Beneyto F, Suso-Vergara S. Acute infection in total knee arthroplasty: diagnosis and treatment. Open Orthop J. 2013;7:197-204. [4] Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272. [5] Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167-193. [6] Gilbert P, Collier PJ, Brown MR. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990;34(10): 1865-1868. [7] Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277-281. [8] Park J, Bauer S, Schmuki P, von der Mark K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009;9(9): 3157-3164. [9] Park J, Bauer S, von der Mark K, et al. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7(6):1686-1691. [10] Popat KC, Leoni L, Grimes CA, et al. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007;28:3188-3197. [11] Brammer KS, Oh S, Cobb CJ, et al. Improved bone-forming functionality on diameter-controlled TiO(2) nanotube surface. Acta Biomater. 2009;5(8):3215-3223. [12] Bjursten LM, Rasmusson L, Oh S, et al. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A. 2010;92(3): 1218-1224. [13] Zhang HZ, Sun Y, Tian A, et al. Improved antibacterial activity and biocompatible on the vancomycin-loaded TiO2 nanotubes: in vivo and in vitro studies, International J Nanomed 2013;8:4379-4389. [14] Cook JL, Scott RD, Long WJ. Late hematogenous infections after total knee arthroplasty: experience with 3013 consecutive total knees. J Knee Surg. 2007;20(1):27-33. [15] Schmalzried TP, Amstutz HC, Au MK, et al. Etiology of deep sepsis in total hip arthrosplasty. The significance of hematogenous and recurrent infections. Clin Orthop Rel Res. 1992;280:200-207.[16] Zhang E, Li F, Wang H, et al. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater Sci Eng C Mater Biol Appl. 2013;33(7):4280-4287. [17] Tian XB, Wang ZM, Yang SQ, et al. Antibacterial copper-containing titanium nitride films produced by dual magnetron sputtering, Surf Coat Technol. 2007;201:8606-8609.[18] Secinti KD, Ayten M, Kahilogullari G, et al. Antibacterial effects of electrically activated vertebral implants. J Clin Neurosci 2008;15(4): 434-439. [19] Wan YZ, Xiong GY, Liang H, et al. Modi?cation of medical metals by ion implantation of copper. Appl Surf Sci. 2007;253:9426-9429. [20] Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res A. 2006;78(3):595-604. [21] Melaiye AYW. Silver and its application as an antimicrobial agent. Expert Opin Ther Pat. 2005;15:125-130. [22] Zheng X, Chen Y, Xie Y, et al. Antibacterial property and biocompatibility of plasma sprayed hydroxyapatite/silver composite coatings. Repentu Jishu Zazhi. 2009;18:463.[23] Azam A, Ahmed AS, Oves M, et al. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int J Nanomedicine. 2012;7:3527-3535. [24] Tsao N, Luh TY, Chou CK, et al. In vitro action of carboxyfullerene. J Antimicrob Chemother. 2002;49(4):641-649. [25] Ercan B, Taylor E, Alpaslan E et al. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology. 2011;22(29):295102. [26] Helmus MN, Gibbons DF, Cebon D. Biocompatibility: meeting a key functional requirement of next-generation medical devices. Toxicol Pathol. 2008;36(1):70-80. [27] Puckett SD, Taylor E, Raimondo T, et al. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 31:706-713. [28] Oh SH, Finõnes RR, Daraio C, et al. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials. 2005;26(24):4938-4943. [29] Kim HM, Himeno T, Kawashita M, et al. Surface potential change in bioactive titanium metal during the process of apatite formation in simulated body fluid. J Biomed Mater Res. 2003;67A(4):1305-1309.[30] Qian T, Wang Y. Micro/nano-fabrication technologies for cell biology. Med Biol Eng Comput. 2010;48(10):1023-1032. [31] Meyer U, Büchter A, Wiesmann HP, et al. Basic reactions of osteoblasts on structured material surfaces. Eur Cell Mater. 2005;9: 39-49. [32] Beutner R, Michael J, Schwenzer B, et al. Biological nano-functionalization of titanium-based biomaterial surfaces: a flexible toolbox. J R Soc Interface. 2010;6;7 Suppl 1:S93-S105.[33] Popat KC, Eltgroth M, Latempa TJ, et al. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28(32):4880-4888. [34] Ren L, Wong HM, Yan CH, et al. Osteogenic ability of Cu-bearing stainless steel. J Biomed Mater Res B Appl Biomater. 2015; 103(7):1433-1444.[35] Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563. [36] Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35(9):780-789. |
| [1] | 张同同, 王中华, 文 杰, 宋玉鑫, 刘 林. 3D打印模型在颈椎肿瘤手术切除与重建中的应用[J]. 中国组织工程研究, 2021, 25(9): 1335-1339. |
| [2] | 周继辉, 李新志, 周 游, 黄 卫, 陈文瑶. 髌骨骨折修复内植物选择的多重问题[J]. 中国组织工程研究, 2021, 25(9): 1440-1445. |
| [3] | 曾燕华, 郝延磊. 许旺细胞体外培养及纯化的系统性综述[J]. 中国组织工程研究, 2021, 25(7): 1135-1141. |
| [4] | 孔令宝, 吕 欣. 胫骨后外侧平台骨折手术治疗中植入物选择与入路对支撑作用的影响[J]. 中国组织工程研究, 2021, 25(6): 942-947. |
| [5] | 徐东紫, 张 婷, 欧阳昭连. 心脏组织工程领域全球专利竞争态势分析[J]. 中国组织工程研究, 2021, 25(5): 807-812. |
| [6] | 吴子健, 胡昭端, 谢有琼, 王 峰, 李 佳, 李柏村, 蔡国伟, 彭 锐. 3D打印技术与骨组织工程研究文献计量及研究热点可视化分析[J]. 中国组织工程研究, 2021, 25(4): 564-569. |
| [7] | 常文辽, 赵 杰, 孙晓亮, 王 锟, 吴国锋, 周 剑, 李树祥, 孙 晗. 人工骨膜的材料选择、理论设计及生物仿生功能[J]. 中国组织工程研究, 2021, 25(4): 600-606. |
| [8] | 刘 旒, 周箐竹, 龚 桌, 刘博言, 杨 斌, 赵 娴. 胶原/无机材料构建组织工程骨的特点及制造技术[J]. 中国组织工程研究, 2021, 25(4): 607-613. |
| [9] | 刘 飞, 崔宇韬, 刘 贺. 局部抗生素递送系统治疗骨髓炎的优势与问题[J]. 中国组织工程研究, 2021, 25(4): 614-620. |
| [10] | 李晓壮, 段 浩, 王伟舟, 唐志宏, 王旸昊, 何 飞. 骨组织工程材料治疗骨缺损疾病在体内实验中的应用[J]. 中国组织工程研究, 2021, 25(4): 626-631. |
| [11] | 张振坤, 李 喆, 李 亚, 王莹莹, 王亚苹, 周馨魁, 马珊珊, 关方霞. 海藻酸盐基水凝胶/敷料在创面愈合中的应用:持续、动态与顺序释放[J]. 中国组织工程研究, 2021, 25(4): 638-643. |
| [12] | 陈佳娜, 邱燕玲, 聂敏海, 刘旭倩. 组织工程支架材料修复口腔颌面部软组织缺损[J]. 中国组织工程研究, 2021, 25(4): 644-650. |
| [13] | 邢 浩, 张永红, 王 栋. 长骨大段骨缺损修复方法的优势与不足[J]. 中国组织工程研究, 2021, 25(3): 426-430. |
| [14] | 舒启航, 廖亦佳, 薛静波, 晏怡果, 王 程. 新型颈椎3D打印多孔椎间融合器的三维有限元分析[J]. 中国组织工程研究, 2021, 25(24): 3810-3815. |
| [15] | 王秋霏, 顾 叶, 彭育沁, 薛 峰, 巨 荣, 朱 锋, 王熠军, 耿德春, 徐耀增. 人工假体磨损颗粒作用下Wnt/β-catenin信号通路对成骨细胞的影响[J]. 中国组织工程研究, 2021, 25(24): 3894-3901. |
Design
.jpg)
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||