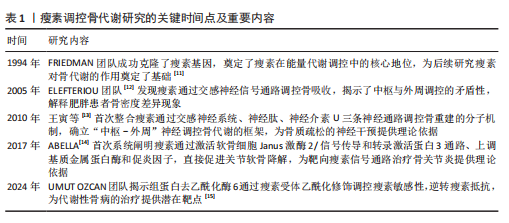
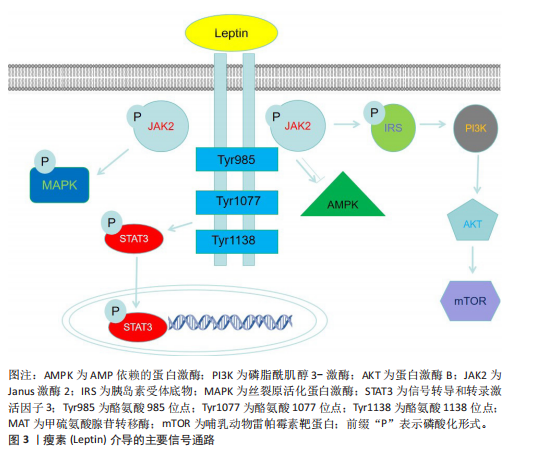
[1] ARJUNAN D, PRASAD TN, DAS L, et al. Osteoporosis and Obesity. Indian J Orthop. 2023;57(Suppl 1):218-224.
[2] KIRK B, FEEHAN J, LOMBARDI G, et al. Muscle, Bone, and Fat Crosstalk: the Biological Role of Myokines, Osteokines, and Adipokines. Curr Osteoporos Rep. 2020; 18(4):388-400.
[3] DUCY P, AMLING M, TAKEDA S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197-207.
[4] TAKEDA S, ELEFTERIOU F, LEVASSEUR R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305-317.
[5] HE Q, QIN R, GLOWACKI J, et al. Synergistic stimulation of osteoblast differentiation of rat mesenchymal stem cells by leptin and 25(OH)D(3) is mediated by inhibition of chaperone-mediated autophagy. Stem Cell Res Ther. 2021;12(1):557.
[6] GRAEF F, WEI Y, GARBE A, et al. Increased cancellous bone mass accompanies decreased cortical bone mineral density and higher axial deformation in femurs of leptin-deficient obese mice. J Mech Behav Biomed Mater. 2024;160:106745.
[7] HU H, LUO S, LAI P, et al. ANGPTL4 binds to the leptin receptor to regulate ectopic bone formation. Proc Natl Acad Sci U S A. 2024;121(1):e2310685120.
[8] MA S, WANG N, ZHANG P, et al. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ. 2021;9:e12293.
[9] MOHAMMADI SM, SANIEE N, BORZOO T, et al. Osteoporosis and Leptin: A Systematic Review. Iran J Public Health. 2024;53(1):93-103.
[10] HANSEN MS, FROST M. Alliances of the gut and bone axis. Semin Cell Dev Biol. 2022;123:74-81.
[11] ZHANG Y, PROENCA R, MAFFEI M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425-432.
[12] ELEFTERIOU F, AHN JD, TAKEDA S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514-520.
[13] 王寅,马丽焱.瘦素对骨重建的神经调节[J].生理科学进展,2010,41(3):229-231.
[14] ABELLA V, SCOTECE M, CONDE J, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100-109.
[15] GUAN D, MEN Y, BARTLETT A, et al. Central inhibition of HDAC6 re-sensitizes leptin signaling during obesity to induce profound weight loss. Cell Metab. 2024;36(4):857-876.
[16] OBRADOVIC M, SUDAR-MILOVANOVIC E, SOSKIC S, et al. Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne). 2021;12:585887.
[17] SOMMER C, VANGBERG KG, MOEN G, et al. Insulin and Body Mass Index Decrease Serum Soluble Leptin Receptor Levels in Humans. J Clin Endocrinol Metab. 2023; 108(5):1110-1119.
[18] GRASSO P. Harnessing the Power of Leptin: The Biochemical Link Connecting Obesity, Diabetes, and Cognitive Decline. Front Aging Neurosci. 2022;14:861350.
[19] FAN X, YUAN W, HUANG W, et al. Recent progress in leptin signaling from a structural perspective and its implications for diseases. Biochimie. 2023;212:60-75.
[20] FAN X, QIN R, YUAN W, et al. The solution structure of human leptin reveals a conformational plasticity important for receptor recognition. Structure. 2024;32(1): 18-23.
[21] HANKIR MK, BRUNEAU M. Periphery-Brain Interactions and Leptin in the Regulation of Whole-Body Energy Metabolism. Nutrients. 2022;14(8):1594.
[22] MANGION D, PACE NP, FORMOSA MM. The relationship between adipokine levels and bone mass-A systematic review. Endocrinol Diabetes Metab. 2023;6(3):e408.
[23] AYED K, NABI L, AKROUT R, et al. Obesity and cancer: focus on leptin. Mol Biol Rep. 2023;50(7):6177-6189.
[24] CHILDS GV, ODLE AK, MACNICOL MC, et al. The Importance of Leptin to Reproduction. Endocrinology (Philadelphia). 2021;162(2):1.
[25] GUO Z, PENG Y, HU Q, et al. The relationship between leptin and periodontitis: a literature review. PeerJ. 2023;11:e16633.
[26] KIERNAN K, MACIVER NJ. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front Immunol. 2020;11:622468.
[27] PATIAL K, MISHRA HP, PAL G, et al. Assessment of Leptin Levels and Their Correlation With the Severity of Obstructive Sleep Apnea Syndrome: A Case-Control Study. Cureus. 2023;15(7):e42028.
[28] VILARIÑO-GARCÍA T, POLONIO-GONZÁLEZ M, PÉREZ-PÉREZ A, et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int J Mol Sci. 2024;25(4):2338.
[29] MUNZBERG H, HEYMSFIELD SB, BERTHOUD H, et al. History and future of leptin: Discovery, regulation and signaling. Metabolism. 2024;161:156026.
[30] YANG Y, WANG Z, GE H, et al. Leptin signaling promotes milk fat synthesis via PI3K/AKT/mTOR/SREBP1 in mammary gland of dairy cow. J Dairy Res. 2024;91(4):433-444.
[31] WEE NKY, DE LIMA TFC, MCGREGOR NE, et al. Leptin receptor in osteocytes promotes cortical bone consolidation in female mice. J Endocrinol. 2022;255(1):25-37.
[32] GAO X, MURPHY MM, PEYER JG, et al. Leptin receptor(+) cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nat Cell Biol. 2023;25(12):1746-1757.
[33] HABERMAN ER, SARKER G, ARÚS BA, et al.Immunomodulatory leptin receptor+ sympathetic perineurial barrier cells protect against obesity by facilitating brown adipose tissue thermogenesis. Immunity (Cambridge, Mass.). 2024;57(1):141-152.
[34] BAKSHI A, SINGH R, RAI U. Trajectory of leptin and leptin receptor in vertebrates: Structure, function and their regulation. Comp Biochem Physiol B Biochem Mol Biol. 2022;257:110652.
[35] XIAO H, LI W, QIN Y, et al. Crosstalk between Lipid Metabolism and Bone Homeostasis: Exploring Intricate Signaling Relationships. Research (Wash D C). 2024;7:447.
[36] YAMAZAKI S, MABUCHI Y, KIMURA T, et al. Activated mesenchymal stem/stromal cells promote myeloid cell differentiation via CCL2/CCR2 signaling. Stem Cell Reports. 2024;19(3):414-425.
[37] ZHAO Y, PENG X, WANG Q, et al. Crosstalk Between the Neuroendocrine System and Bone Homeostasis. Endocr Rev. 2024;45(1): 95-124.
[38] TSUCHIYA H, FUJIO K. Emerging role of leptin in joint inflammation and destruction. Immunol Med. 2022;45(1):27-34.
[39] STEFANAKIS K, UPADHYAY J, RAMIREZ-CISNEROS A, et al. Leptin physiology and pathophysiology in energy homeostasis, immune function, neuroendocrine regulation and bone health. Metabolism. 2024;161:156056.
[40] PRASAD P, CANCELAS JA. From Marrow to Bone and Fat: Exploring the Multifaceted Roles of Leptin Receptor Positive Bone Marrow Mesenchymal Stromal Cells. Cells. 2024;13(11):910.
[41] ANSARIN A, MAHDAVI A M, JAVADIVALA Z, et al. The cross-talk between leptin and circadian rhythm signaling proteins in physiological processes: a systematic review. Mol Biol Rep. 2023;50(12):10427-10443.
[42] DEEPIKA F, BATHINA S, ARMAMENTO-VILLAREAL R. Novel Adipokines and Their Role in Bone Metabolism: A Narrative Review. Biomedicines. 2023;11(2):644.
[43] APPELT J, TSITSILONIS S, OTTO E, et al. Mice Lacking the Calcitonin Receptor Do Not Display Improved Bone Healing. Cells (Basel, Switzerland). 2021;10(9):2304.
[44] GARBE A, GRAEF F, APPELT J, et al. Leptin Mediated Pathways Stabilize Posttraumatic Insulin and Osteocalcin Patterns after Long Bone Fracture and Concomitant Traumatic Brain Injury and Thus Influence Fracture Healing in a Combined Murine Trauma Model. Int J Mol Sci. 2020;21(23):9144.
[45] LI J, GAO Y, YU T, et al. Obesity and leptin influence vitamin D metabolism and action in human marrow stromal cells. J Steroid Biochem Mol Biol. 2020;198:105564.
[46] NIWCZYK O, GRYMOWICZ M, SZCZESNOWICZ A, et al. Bones and Hormones: Interaction between Hormones of the Hypothalamus, Pituitary, Adipose Tissue and Bone. Int J Mol Sci. 2023;24(7):6840.
[47] HSU C, KAO C, YANG C, et al. Leptin Promotes the Expression of Pro-inflammatory Mediator Genes but Does Not Alter Osteoclastogenesis and Early Stage Differentiation of Osteoblasts. J Physiol Investig. 2024;67(6):355-363.
[48] MISCH M, PUTHANVEETIL P. The Head-to-Toe Hormone: Leptin as an Extensive Modulator of Physiologic Systems. Int J Mol Sci. 2022;23(10):5439.
[49] SHI H, CHEN M. The brain-bone axis: unraveling the complex interplay between the central nervous system and skeletal metabolism. Eur J Med Res. 2024;29(1):317.
[50] 汪青,林华.瘦素在骨性关节炎病理机制中的作用[J].中华骨质疏松和骨矿盐疾病杂志,2022,15(4):435-439.
[51] CORDERO-BARREAL A, GONZALEZ-RODRIGUEZ M, RUIZ-FERNANDEZ C, et al. An Update on the Role of Leptin in the Immuno-Metabolism of Cartilage. Int J Mol Sci. 2021;22(5):2411.
[52] BEGHINI M, BRANDT S, KORBER I, et al. Serum IGF1 and linear growth in children with congenital leptin deficiency before and after leptin substitution. Int J Obes (Lond). 2021;45(7):1448-1456.
[53] XIAO Y, HAN C, WANG Y, et al. Interoceptive regulation of skeletal tissue homeostasis and repair. Bone Res. 2023;11(1):48.
[54] BARRIOS V, FRAGO LM, CANELLES S, et al. Leptin Modulates the Response of Brown Adipose Tissue to Negative Energy Balance: Implication of the GH/IGF-I Axis. Int J Mol Sci. 2021;22(6):2827.
[55] SALAMANNA F, CONTARTESE D, VERONESI F, et al. Osteoporosis Preclinical Research: A Systematic Review on Comparative Studies Using Ovariectomized Sheep. Int J Mol Sci. 2022;23(16):8904.
[56] ZHU S, CHEN W, MASSON A, et al. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024;10(1):71.
[57] PERAKAKIS N, MANTZOROS CS. Evidence from clinical studies of leptin: current and future clinical applications in humans. Metabolism. 2024;161(6):156053.
[58] KARSENTY G, KHOSLA S. The crosstalk between bone remodeling and energy metabolism: A translational perspective. Cell Metab. 2022;34(6):805-817.
[59] CAO L, CHOI EY, LIU X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3): 324-338.
[60] LV X, GAO F, CAO X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 2022;34(12):1914-1931.
[61] ZENG W, YANG F, SHEN WL, et al. Interactions between central nervous system and peripheral metabolic organs. Sci China Life Sci. 2022;65(10):1929-1958.
[62] LIU H, LIU L, ROSEN CJ. Bone Marrow Adipocytes as Novel Regulators of Metabolic Homeostasis: Clinical Consequences of Bone Marrow Adiposity. Curr Obes Rep. 2025;14(1):9.
[63] PERAKAKIS NM, FARR OMP, MANTZOROS CSM. Leptin in Leanness and Obesity. J Am Coll Cardiol. 2021;77(6):745-760.
[64] PEREIRA S, CLINE DL, GLAVAS MM, et al. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr Rev. 2021; 42(1):1-28.
[65] PICÓ C, PALOU M, POMAR CA, et al. Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord. 2022;23(1):13-30.
[66] ASGARI R, CACERES-VALDIVIEZO M, WU S, et al. Regulation of energy balance by leptin as an adiposity signal and modulator of the reward system. Mol Metab. 2025; 91:102078.
[67] CHOI W, KIM J, KANG H, et al. Interactive Effects of Serum Leptin Levels and Physical Comorbidity on the Pharmacotherapeutic Response of Depressive Disorders. Clin Psychopharmacol Neurosci. 2022;20(4): 662-674.
[68] LI C, PI G, LI F. The Role of Intestinal Flora in the Regulation of Bone Homeostasis. Front Cell Infect Microbiol. 2021;11:579323.
[69] ZHANG B, CUI J, ZHANG X, et al. Autophagy: regulating the seesaw of bone-fat balance. Front Cell Dev Biol. 2025;13:1465092.
[70] LIN Z, XIONG S, LIN Y, et al. Impact of leptin or melatonin on Sema4D overexpression-related bone metabolism. J Orthop Surg Res. 2023;18(1):285.
[71] WANG N, MA S, FU L. Gut Microbiota Dysbiosis as One Cause of Osteoporosis by Impairing Intestinal Barrier Function. Calcif Tissue Int. 2022;110(2):225-235.
[72] DIMAI HP, MUSCHITZ C, AMREIN K, et al. [Osteoporosis-Definition, risk assessment, diagnosis, prevention and treatment (update 2024): Guidelines of the Austrian Society for Bone and Mineral Research]. Wien Klin Wochenschr. 2024;136(Suppl 16): 599-668.
[73] ZHANG Y, LI Y, LU P, et al. The modulatory effect and implication of gut microbiota on osteoporosis: from the perspective of “brain-gut-bone” axis. Food Funct. 2021; 12(13):5703-5718.
[74] OZAKI D, KUBOTA R, MAENO T, et al. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32(1):145-156.
[75] INDRIO F, SALATTO A. Gut Microbiota-Bone Axis. Ann Nutr Metab. 2025;81(Suppl 1): 47-56.
[76] LIU Q, ZHU Y, LI G, et al. Irisin ameliorates myocardial ischemia-reperfusion injury by modulating gut microbiota and intestinal permeability in rats. PLoS One. 2023;18(9): e0291022.
[77] VAN SON J, KOEKKOEK LL, LA FLEUR SE, et al. The Role of the Gut Microbiota in the Gut-Brain Axis in Obesity: Mechanisms and Future Implications. Int J Mol Sci. 2021; 22(6):2993.
[78] HOSSEINIFARD E, BAVAFA-VALENLIA K, SAGHAFI-ASL M, et al. Antioxidative and Metabolic Effects of Lactobacillus plantarum, Inulin, and Their Synbiotic on the Hypothalamus and Serum of Healthy Rats. Nutr Metab Insights. 2020;13: 1178638820925092.
[79] YUAN L, LI Y, CHEN M, et al. Antihypertensive Activity of Milk Fermented by Lactiplantibacillus plantarum SR37-3 and SR61-2 in L-NAME-Induced Hypertensive Rats. Foods. 2022;11(15):2332.
[80] GEWITZ A, MENDELL J, WANG Y, et al. Pharmacokinetics and pharmacodynamics of mibavademab (a leptin receptor agonist): Results from a first-in-human phase I study. Clin Transl Sci. 2024;17(4):e13762. |