中国组织工程研究 ›› 2026, Vol. 30 ›› Issue (12): 3109-3126.doi: 10.12307/2026.735
• 组织构建综述 tissue construction review • 上一篇 下一篇
金属暴露对神经退行性疾病的影响及其神经毒理学机制
刘 圳1,周 晶2,3,4,5,6,杨 丹2,3,4,5,刘 婉2,3,4,5,赵 焰2,3,4,5,6,罗昱君2,3,4,5,曹必伟2,3,4,5,6,7
- 1湖北中医药大学针灸骨伤学院,湖北省武汉市 430065;2湖北省中医院,湖北省武汉市 430061;3湖北时珍实验室,湖北省武汉市 430065;4湖北中医药大学附属医院,湖北省武汉市 430061;5湖北省中医院推拿科/康复医学科,湖北省武汉市 430061;6湖北中医药大学第一临床学院,湖北省武汉市 430065;7中医肝肾研究及应用湖北重点实验室(湖北省中医院),湖北省武汉市 430061
-
收稿日期:2025-07-09接受日期:2025-08-30出版日期:2026-04-28发布日期:2025-09-30 -
通讯作者:曹必伟,硕士生导师,副教授,副主任医师,湖北省中医院,湖北省武汉市 430061;湖北时珍实验室,湖北省武汉市 430065;湖北中医药大学附属医院,湖北省武汉市 430061;湖北省中医院推拿科/康复医学科,湖北省武汉市 430061;湖北中医药大学第一临床学院,湖北省武汉市 430065;中医肝肾研究及应用湖北重点实验室(湖北省中医院),湖北省武汉市 430061 并列通讯作者:罗昱君,在读博士,主治医师,湖北省中医院,湖北省武汉市 430061;湖北时珍实验室,湖北省武汉市 430065;湖北中医药大学附属医院,湖北省武汉市 430061;湖北省中医院推拿科/康复医学科,湖北省武汉市 430061 -
作者简介:刘圳,男,2000年生,湖南省株洲市人,汉族,湖北中医药大学在读硕士,主要从事推拿学理论与临床应用研究及推拿技术治疗运动神经元病的研究。 -
基金资助:2023年湖北省自然科学基金计划(联合基金项目)(2023AFD165),项目负责人:罗昱君;2023年湖北省自然科学基金计划(联合基金项目)(2023AFD128),项目负责人:周晶
Impact of metal exposure on neurodegenerative diseases: advances in understanding neurotoxicological mechanisms
Liu Zhen1, Zhou Jing2, 3, 4, 5, 6, Yang Dan2, 3, 4, 5, Liu Wan2, 3, 4, 5, Zhao Yan2, 3, 4, 5, 6, Luo Yujun2, 3, 4, 5, Cao Biwei2, 3, 4, 5, 6, 7
- 1School of Acupuncture-Moxibustion and Orthopedics, Hubei University of Chinese Medicine, Wuhan 430065, Hubei Province, China; 2Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan 430061, Hubei Province, China; 3Hubei Sizhen Laboratory, Wuhan 430065, Hubei Province, China; 4Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; 5Department of Tuina and Rehabilitation Medicine, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan 430061, Hubei Province, China; 6The First Clinical Medical School, Hubei University of Chinese Medicine, Wuhan 430065, Hubei Province, China; 7Hubei Key Laboratory of Traditional Chinese Medicine Research and Application on Liver and Kidney (Hubei Provincial Hospital of Traditional Chinese Medicine), Wuhan 430061, Hubei Province, China
-
Received:2025-07-09Accepted:2025-08-30Online:2026-04-28Published:2025-09-30 -
Contact:Cao Biwei, Master’s supervisor, Associate professor, Associate chief physician, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan 430061, Hubei Province, China; Hubei Sizhen Laboratory, Wuhan 430065, Hubei Province, China; Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; Department of Tuina and Rehabilitation Medicine, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan 430061, Hubei Province, China; The First Clinical Medical School, Hubei University of Chinese Medicine, Wuhan 430065, Hubei Province, China; Hubei Key Laboratory of Traditional Chinese Medicine Research and Application on Liver and Kidney (Hubei Provincial Hospital of Traditional Chinese Medicine), Wuhan 430061, Hubei Province, China Co-corresponding author: Luo Yujun, PhD candidate, Attending physician, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan 430061, Hubei Province, China; Hubei Sizhen Laboratory, Wuhan 430065, Hubei Province, China; Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; Department of Tuina and Rehabilitation Medicine, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan 430061, Hubei Province, China -
About author:Liu Zhen, MS candidate, School of Acupuncture-Moxibustion and Orthopedics, Hubei University of Chinese Medicine, Wuhan 430065, Hubei Province, China -
Supported by:2023 Hubei Provincial Natural Science Foundation Program (Joint Fund Project), Nos. 2023AFD165 (to LYJ) and 2023AFD128 (to ZJ)
摘要:
文题释义:
金属暴露:指生物体通过环境或职业途径与游离态、离子态或化合物形式的金属元素发生接触,导致金属元素在生物体内积累并引发潜在毒理学效应的过程。根据金属的生物学功能可将其分为必需金属和非必需金属。暴露的毒理效应取决于金属的化学形态、剂量-反应关系及暴露窗口。
神经退行性疾病:是一类以中枢神经系统或周围神经系统神经元数量减少、结构损伤或功能障碍为主要特征的疾病,这类疾病通常表现为慢性、不可逆的病理过程,导致认知功能、运动能力或感觉功能的逐渐丧失。
背景:研究表明,金属暴露与神经退行性疾病的发生和发展关系密切。
目的:系统、全面地阐述金属暴露对神经退行性疾病的影响,总结金属暴露对神经退行性疾病作用的神经毒理学机制。
方法:由第一作者应用计算机检索中国知网、Web of Science和PubMed数据库建库至2025年2月收录的文献,英文检索词为“Metal exposure,Manganese,Iron,Zinc,Copper,Cadmium,Lead,Aluminum,Neurodegenerative diseases,Alzheimer’s disease,Parkinson‘s disease,Multiple sclerosis,Amyotrophic lateral sclerosis,Huntington’s disease”等,中文检索词为“金属暴露,锰,铁,锌,铜,镉,铅,铝,神经退行性疾病,阿尔茨海默病,帕金森病,多发性硬化,肌萎缩侧索硬化,亨廷顿舞蹈病”等。根据入选标准,最终纳入204篇文献进行综述分析。
结果与结论:①锰暴露通过线粒体氧化应激、神经炎症反应、蛋白质稳态紊乱多机制系统性驱动神经退行性病变过程;②铁暴露引起的游离铁异常蓄积可触发线粒体功能障碍、蛋白稳态失衡、神经炎症反应和表观遗传失调,同时神经炎症形成的炎症微环境会加剧游离铁异常蓄积,进而导致神经元凋亡、路易小体沉积、淀粉样病变及血脑屏障破坏等病理过程,推动阿尔茨海默病与帕金森病等神经退行性疾病发生发展;③锌超载通过双路径损害神经元:胞内锌内流引发内质网应激,导致钙失衡和线粒体功能障碍,胞外突触锌激活活性氧-谷氨酸毒性轴,二者经p38丝裂原活化蛋白激酶/c-Jun氨基末端激酶通路放大凋亡信号,协同Toll 样受体4/核因子κB介导的神经炎症共同驱动阿尔茨海默病等神经退行性病变;④铜通过芬顿反应介导的氧化应激、蛋白异常聚集、铜死亡以及神经递质失衡发挥神经毒性,同时破坏血脑屏障加剧铜蓄积,形成自我强化的神经毒性网络,驱动神经退行性疾病的病理进程;⑤镉通过诱导氧化应激、线粒体功能障碍、细胞凋亡干扰钙稳态及神经递质代谢,激活炎症反应与破坏血脑屏障,共同导致神经退行性病变;⑥铅通过劫持金属转运蛋白引发氧化应激与线粒体凋亡,上调兰尼碱受体致钙超载抑制突触可塑性,通过表观遗传失调加剧β-淀粉样蛋白沉积及突触损伤,推动神经退行性病变;⑦铝通过破坏铁代谢诱发铁超载和铁死亡,干扰乙酰胆碱代谢损害胆碱能系统,抑制胰岛素受体底物1/磷脂酰肌醇3-激酶/蛋白激酶B通路导致tau蛋白异常磷酸化和β-淀粉样蛋白沉积,激活c-Jun氨基末端激酶/NOD样受体热蛋白结构域相关蛋白3通路引发坏死性凋亡与细胞焦亡,协同诱导神经炎症、突触可塑性损伤及神经元凋亡最终引发认知功能障碍和神经退行性病变;⑧金属植入物释放的有毒离子(如过量锌、铁)可能引发神经细胞氧化应激、兴奋性毒性及线粒体功能障碍,从而干扰神经再生,可通过优化支架孔隙率、力学性能及表面涂层技术(如银纳米颗粒)来抑制离子溶出;⑨金属螯合剂通过结合游离金属离子降低神经毒性,不仅可清除阿尔茨海默病中的铁离子沉积、溶解β-淀粉样蛋白缠结,还能与干细胞移植协同减轻创伤性脑损伤局部的氧化应激和炎症,提升神经修复效果;⑩干预金属毒性的组织工程新兴策略包括开发靶向递送系统(如负载乙二胺四乙酸钙纳米颗粒穿越血脑屏障清除重金属)和智能响应材料(如金属离子敏感水凝胶动态调控金属离子释放),以精准维持神经微环境中必需金属元素的平衡。
https://orcid.org/0009-0000-5221-9782(刘圳)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
刘 圳, 周 晶, 杨 丹, 刘 婉, 赵 焰, 罗昱君, 曹必伟. 金属暴露对神经退行性疾病的影响及其神经毒理学机制[J]. 中国组织工程研究, 2026, 30(12): 3109-3126.
Liu Zhen, Zhou Jing, Yang Dan, Liu Wan, Zhao Yan, Luo Yujun, Cao Biwei. Impact of metal exposure on neurodegenerative diseases: advances in understanding neurotoxicological mechanisms[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3109-3126.
2.2 金属暴露与神经退行性疾病的关联
2.2.1 锰与神经退行性疾病 锰(Mn)是一种人体必需微量金属元素,可以从谷物、坚果、豆类、菠萝等食物中摄取,在消化道被吸收;此外,人体还可通过皮肤直接接触或经呼吸道吸入空气中的锰。锰参与人体多种代谢和酶促反应,尤其在大脑中发挥重要作用[33]。适量的锰元素对神经细胞功能至关重要,它参与谷氨酸和γ-氨基丁酸等神经递质的传递、抗氧化应激反应、细胞周期调控、DNA损伤修复或凋亡触发等过程[30,34-38]。但研究表明,环境污染、职业暴露以及饮食摄入等途径导致的锰过量暴露可引起大脑等部位锰水平异常升高[39-41],破坏锰稳态,进而可能影响神经细胞功能[42-45]。
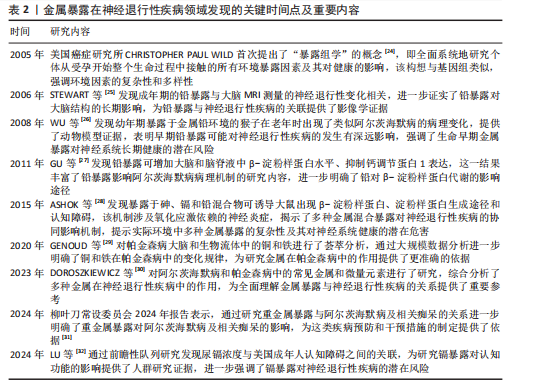
锰过量暴露后,当它在体内积累超过特定“阈值”时,首先会在神经细胞线粒体中富集,锰在线粒体中与特定底物(如琥珀酸和苹果酸)结合后,能够激活氧化应激相关的p38丝裂原活化蛋白激酶和c-Jun氨基末端激酶信号通路,进而导致氧化磷酸化异常,诱导活性氧的产生,增加过氧化氢的生成,同时降低耗氧量并提高细胞外酸化率,最终导致线粒体功能障碍[46-48]。随之而来的线粒体功能障碍通过破坏膜电位(ΔΨm)导致ATP生成不足、钙超载及活性氧累积,同时促凋亡因子(如细胞色素c)经线粒体通透性转换孔开放或Bax/Bak激活释放至胞质,与凋亡酶激活因子1形成凋亡小体激活caspase级联反应,最终切割关键蛋白引发神经元凋亡[49]。例如,在一项使用N27大鼠来源中脑多巴胺能神经元细胞的实验中,研究者发现高浓度的锰暴露激活了促多巴胺能神经元细胞凋亡的相关蛋白质,如线粒体细胞色素c的释放、caspase-3的激活、蛋白激酶Cδ的激活以及DNA的片段化[17,50]。
此外,锰还通过诱发神经炎症反应加剧神经损伤,影响神经胶质细胞的功能而产生神经毒性[51]。作为中枢神经系统中的固有免疫细胞,小胶质细胞面对锰水平升高的情况时会释放促炎细胞因子白细胞介素6、肿瘤坏死因子α和白细胞介素1β以触发并维持炎症反应[52],而与小胶质细胞有着协同免疫作用的星形胶质细胞则激活了p38丝裂原活化蛋白激酶和核因子κB通路,进一步加剧了神经炎症[53]。总体而言,锰诱导的胶质细胞活化可导致活性氧和一氧化氮的产生,使炎性细胞因子在周围组织中扩散,从而加重炎症反应。因此,基底神经节内小胶质细胞的异常活化被认为是锰神经毒性相关神经炎症过程中的关键环节[54]。
锰过量暴露还通过多层级机制协同破坏蛋白质稳态产生神经毒性。在分子层面,锰离子通过竞争性结合干扰金属伴侣蛋白功能,破坏α-突触核蛋白等金属依赖性蛋白的正确折叠[55];锰离子特异性激活的非受体酪氨酸激酶还促使α-突触核蛋白发生病理性酪氨酸磷酸化,显著增强蛋白寡聚化倾向[56]。在亚细胞层面,锰暴露持续激活内质网未折叠蛋白反应,削弱分子伴侣系统的修复能力,同时破坏高尔基体-内体运输网络,抑制蛋白酶体去泛素化及自噬体成熟,最终导致错误折叠蛋白在胞内累积[57]。更关键的是,锰通过调控RAS致癌基因家族Rab27a/b基因介导的外泌体分泌,促使异常蛋白在神经元与胶质细胞间扩散,而星形胶质细胞摄取的病理性蛋白可激活NLRP3炎症小体,释放的炎性因子通过核因子κB通路进一步加剧神经元内蛋白折叠紊乱,形成自我放大的恶性循环[58-61]。这些异常折叠蛋白最终组装成具有膜穿孔能力的“孔道样”寡聚体,引发钙稳态失衡和线粒体损伤,促使路易小体在黑质多巴胺能神经元中沉积,系统性推动帕金森病的病理进程(图4)。
目前主要应用螯合剂治疗锰暴露导致的神经毒性,乙二胺四乙酸钙作为治疗锰中毒的传统合成螯合剂,通过螯合锰经尿排出。但乙二胺四乙酸钙治疗的局限明显:疗效个体差异大,穿透血脑屏障能力差,疗效低,长期使用或不当使用还可能螯合并排出其他必需金属元素(如锌、铜),甚至存在增加体内其他潜在有毒金属生物利用度的风险[51]。前瞻性的治疗策略则着眼于开发新型天然或合成化合物,例如,来自植物Echium amoenum(紫草科,俗称红羽毛)的提取物,研究表明它能有效抑制锰诱导的神经毒性,关键机制之一在于抑
制促炎转录因子c-Jun和核因子κB向细胞核的转位,从而显著减轻神经炎症反应[35]。
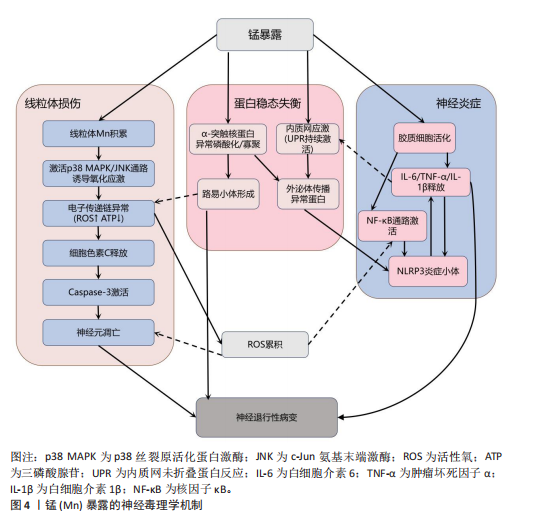
2.2.2 铁与神经退行性疾病 铁(Fe)是维持神经系统正常功能不可或缺的微量元素,也是人类大脑中含量最丰富的过渡金属离子。铁主要从肉类、蔬菜和豆类中等食物获取,并在消化道被吸收;此外,人体还可通过空气从呼吸道吸入铁[62]。铁在脑发育和神经元活动中发挥着关键作用,它作为多种酶的辅因子,参与线粒体呼吸链电子传递、DNA合成及髓鞘形成等重要生理过程[63-64]。铁还通过调控酪氨酸羟化酶活性影响多巴胺合成,并调节突触可塑性[18,65]。然而,当脑内铁稳态失衡导致游离铁异常蓄积时,会引发一系列神经毒性级联反应,见图5。
当游离铁异常蓄积时,首先通过芬顿反应催化产生高活性羟自由基,触发氧化应激反应链[66],这一过程不仅导致神经元膜脂质过氧化和线粒体膜电位崩溃,而且活性氧的增加还会促进参与形成凋亡小体的细胞色素c释放,激活caspase凋亡通路,最终导致神经元凋亡[67]。与此同时,过量的铁离子直接与α-突触核蛋白相互作用,诱导其构象朝β-折叠结构转变、抑制泛素-蛋白酶体系统,降低错误折叠蛋白清除效率,最终促进路易小体等病理性沉积的形成[68-69]。
在表观遗传层面,铁过载通过干扰DNA甲基转移酶和甲基胞嘧啶双加氧酶之间的动态平衡,造成全基因组甲基化水平下降,这种表观遗传失调进而促进tau蛋白过度磷酸化及阿尔茨海默病相关淀粉样前体蛋白基因异常表达,两者皆是阿尔茨海默病的致病因素,共同推动阿尔茨海默病病理进程[70-71]。
更值得注意的是,小胶质细胞的铁沉积会激活Toll 样受体4/核因子κB通路或NLRP3炎症小体,使白细胞介素6、白细胞介素1β等促炎细胞因子分泌量激增,这种慢性神经炎症环境不仅破坏血脑屏障,还通过转铁蛋白受体介导的正反馈循环进一步加
剧脑铁蓄积[72-74]。
目前针对铁蓄积所致神经毒性的治疗策略,主要集中于抗氧化剂和铁螯合剂两类。抗氧化剂(如维生素E、维生素K、α-硫辛酸和N-乙酰半胱氨酸)通过中和自由基并抑制脂质过氧化,减轻铁诱导的氧化应激及细胞损伤。铁螯合剂(如去铁胺、去铁酮)则通过结合铁并促进其排泄,减少细胞内铁积累[75]。同时,天然化合物槲皮素也展现出潜力,它能激活核因子E2相关因子2蛋白、诱导血红素氧合酶1等靶基因表达,有效防止线粒体活性氧过度产生,并降低丙二醛与脂质活性氧水平,从而抑制脂质过氧
化[76]。
2.2.3 锌与神经退行性疾病 锌(Zn)是中枢神经系统中含量第二高的过渡金属元素,在突触传递、神经可塑性、酶活性调控和抗氧化防御等生理过程中发挥关键作用[77-78]。然而,锌的稳态失衡(尤其是过量积累)可引发显著的神经毒性反应,该机制涉及多种分子通路和细胞过程。
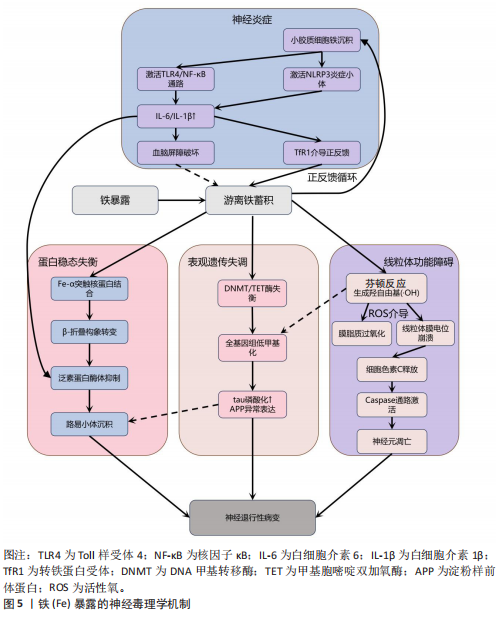
锌过量通过双重途径引发神经元损伤,产生神经毒性。在胞内途径中,锌经电压门控钙离子通道/锌铁转运蛋白通道进入神经元,抑制钙泵并激活兰尼碱受体,诱导内质网应激,增加细胞外钙内流,扰乱细胞内钙稳态,导致钙超载及线粒体功能障碍(表现为ATP耗竭、活性氧激增),线粒体活性氧的增加进一步导致线粒体膜电位下降,诱导线粒体通透性转换孔的开放,致使细胞色素c从线粒体释放,从而触发caspase-3介导的凋亡途径[79]。在胞外途径中,突触锌激活烟酰胺腺嘌呤二核苷酸磷酸氧化酶并产生活性氧,同时锌通过抑制N-甲基-D-天冬氨酸受体甘氨酸位点引发谷氨酸释放增加,这两种效应协同作用共同诱发氧化损伤和兴奋性毒性[80-81]。
值得注意的是,上述胞内和胞外途径均能激活p38丝裂原活化蛋白激酶和c-Jun氨基末端激酶信号通路调控Bax与Bcl-2表达失衡,激活线粒体介导的内源性细胞凋亡途径,从而加速神经元退变[82]。此外,过量锌还能激活Toll样受体4/核因子κB通路,促使胶质细胞释放肿瘤坏死因子α、白细胞介素1β等促炎因子,破坏血脑屏障完整性,并形成炎症-氧化应激正反馈环路[83]。总体而言,锌过量通过这些多层次的交叉作用机制在阿尔茨海默病等神经退行性疾病中形成毒性级联放大效应,见图6。
目前治疗过量锌诱导的神经退行性病变主要依赖锌螯合剂,这类药物通过结合锌离子降低体内锌水平,减轻锌离子对神经细胞的毒性。研究证实,铜-锌螯合剂能显著抑制阿尔茨海默病转基因小鼠大脑中β-淀粉样蛋白的积累,并且起效迅速[84]。
2.2.4 铜与神经退行性疾病 铜(Cu)是人体必需的微量元素,作为多种酶的辅因子在生理过程中发挥着重要作用,包括线粒体呼吸链的电子传递、抗氧化酶的活性调节、神经兴奋性平衡、突触可塑性和神经递质合成等[85-86]。
然而,过量铜蓄积会引发多重神经毒性机制,最终导致神经元损伤和神经退行性疾病(如阿尔茨海默病、帕金森病等)。
铜的核心毒性机制始于通过芬顿反应催化产生的活性氧,例如羟基自由基(?OH),这些自由基直接攻击细
胞膜脂质、蛋白质和DNA,同时耗竭谷胱甘肽等抗氧化分子,加剧氧化应激并诱导线粒体功能障碍,进而激活神经元凋亡通路,最终导致神经元程序性死亡[87-91]。
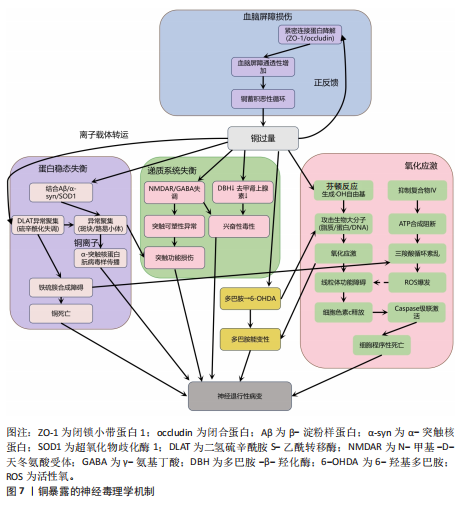
其次,铜与β-淀粉样蛋白、α-突触核蛋白、超氧化物歧化酶1等神经蛋白结合导致其构象改变和异常聚集,形成淀粉样斑块或路易小体,直接破坏突触功能,并且铜离子推动α-突触核蛋白的朊病毒样传播,共同导致神经退行性病变[20,92-95]。线粒体功能障碍在此过程中进一步恶化,过量铜抑制呼吸复合物Ⅳ活性,阻断ATP合成并引发能量代谢崩溃,同时激增的活性氧促进细胞色素c释放激活caspase级联反应,推动程序性细胞死亡[96-97]。而且胞外的铜可通过铜离子载体(如伊利司莫)将胞外铜转运至细胞内,靶向结合三羧酸循环中的硫辛酰化线粒体酶(如二氢硫辛酰胺S-乙酰转移酶),引发其构象改变与异常聚集。铁氧还蛋白1/硫辛酸合成酶调控的硫辛酰化过程失调时,不仅加剧蛋白聚集,还干扰铁硫簇(Fe-S)合成。这些级联效应引发蛋白毒性应激,最终导致神经元铜死亡[98]。
此外,铜还通过协同作用机制发挥双重神经毒性效应。首先,铜离子干扰多巴胺代谢,促使其氧化生成神经毒素6-羟基多巴胺并诱导脂质过氧化,产生氧化应激,两者协同加剧多巴胺能变性[99];其次,铜减少了细胞内将多巴胺转化为去甲肾上腺素的多巴胺-β-羟化酶浓度,抑制去甲肾上腺素合成,导致神经递质系统失衡[100];并且铜对N-甲基-D-天冬氨酸受体和γ-氨基丁酸能信号通路的调控异常,进一步扰乱突触可塑性和神经兴奋性平衡,加速神经退行性病变[101-102]。
慢性铜暴露还可通过诱导血脑屏障内皮细胞产生活性氧,破坏紧密连接蛋白如闭锁小带蛋白1和闭合蛋白,增加血脑屏障通透性,使外周铜更易渗透至中枢神经系统,形成铜蓄积的恶性循环[103]。
上述多通路交互作用共同推动神经退行性疾病的病理进程,见图7。
尽管早期铜螯合剂治疗的疗效有限,但近期研究发现8-羟基喹啉-2-羧醛异烟肼展现出潜力,这种中等亲和力的金属螯合剂能有效竞争结合铜离子(Cu(Ⅰ)/Cu(Ⅱ)),抑制α-突触核蛋白聚集,并能安全穿透血脑屏障[104]。
另一方面,铜螯合剂TTM 通过显著抑制肠道铜吸收并降低循环游离铜水平,减轻铜对肝、脑等器官的毒性。铜蛋白结合剂WTX101在临床试验中能有效控制铜诱导的神经症状并显著改善患者状况[105]。
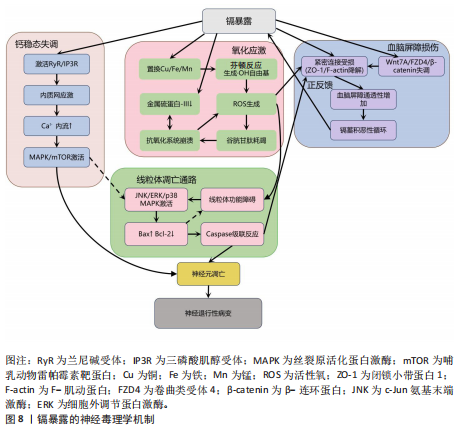
2.2.5 镉与神经退行性疾病 镉(Cd)是一种天然存在的水溶性重金属[106],在人体内没有已知的生理功能,并且被国际癌症研究机构(International Agency for Research on Cancer,IARC)归类为Ⅰ组致癌物[107]。镉作为环境污染物,它的来源涵盖多种自然和人为活动,包括化石燃料燃烧、采矿、电池和颜料的生产以及植物生长等过程[21]。摄入受镉污染的食物和吸入含镉气溶胶是主要的暴露途径[108]。值得注意的是,尽管在生理层面缺乏必需的生理功能,但镉仍能穿透血脑屏障并在特定脑区形成蓄积,镉进入大脑后诱导的神经毒性反应,与氧化应激、DNA 损伤、内质网应激、细胞自噬、神经炎症、血脑屏障受损等有关,影响了阿尔茨海默病、帕金森病、肌萎缩侧索硬化症等神经退行性疾病的发生发展[109-110]。
氧化应激是镉诱导神经毒性的核心机制之一[111]。镉作为一种非氧化还原活性金属,可从蛋白质的金属结合位点置换必需氧化还原金属(铜、铁、锰),并通过芬顿反应催化过氧化氢分解产生破坏性的活性氧,从而诱导氧化应激。持续的氧化应激会造成线粒体功能障碍,并激活c-Jun氨基末端激酶/细胞外调节蛋白激酶 /p38 丝裂原活化蛋白激酶通路,导致Bcl-2表达下调与Bax蛋白、裂解
的caspase-3的表达上调,标志着线粒体介导的内源性细胞凋亡途径被激活[112-113]。此外,镉对巯基(-SH)的高亲和力使它能够与谷胱甘肽和金属硫蛋白等分子特异性结合,从而削弱细胞的抗氧化防御机制。需强调的是,无论是急性高水平还是长期低水平镉暴露均会破坏机体的抗氧化系统[114],引发持续的氧化应激,进而导致抗氧化分子谷胱甘肽耗竭。例如,镉暴露可诱导幼年大鼠产生氧化应激依赖性神经炎症,损害大鼠神经发育;更值得注意的是,铅、镉和砷的混合暴露会通过协同作用显著增强这种神经毒性效应[28]。
镉还通过影响金属硫蛋白和细胞内金属稳态来增强它的神经毒性[115-116]。其中,金属硫蛋白Ⅲ作为一种重要的神经元金属结合蛋白,能够通过螯合细胞内的游离镉离子来减轻镉的毒性。然而金属硫蛋白Ⅲ的表达在长期镉暴露中被显著下调,这种现象与在阿尔茨海默病患者的脑中观察到的金属硫蛋白Ⅲ减少相一致[117],这种金属硫蛋白的缺乏进一步促进了神经元凋亡[118]。与此同时,镉激活兰尼碱受体和三磷酸肌醇受体通路,诱导内质网应激,增加细胞外钙内流,扰乱细胞内钙稳态,紊乱的钙信号进而激活丝裂原活化蛋白激酶/哺乳动物雷帕霉素靶蛋白信号通路,导致神经元凋亡[119-121]。
此外,镉引发的金属稳态失衡还与血脑屏障损伤形成恶性循环:一方面,镉能够增加脑内皮细胞中的活性氧,诱导内质网应激并激活caspase-3介导的凋亡途径,从而破坏血脑屏障的关键结构组分,包括紧密连接蛋白、细胞骨架(F-肌动蛋白)和波形蛋白结构,最终增加血脑屏障的通透性;另一方面,镉还通过干扰Wnt7A/卷曲类受体4/β-连环蛋白信号通路使紧密连接受损和黏附连接形成,进一步诱导血脑屏障功能障碍[122-124]。这些多层次的作用(从氧化应激、金属稳态失调、内质网应激到血脑屏障破坏)共同加剧了镉的神经毒性,并最终促使神经退行性病变的发生与发展,见图8。
当前镉神经毒性的治疗主要聚焦于螯合剂和抗氧化剂。研究表明,乙二胺四乙酸钙螯合治疗能有效清除体内镉等有毒金属,改善患者症状[125]。另一方面,降糖药利格列汀凭借抗氧化、抗凋亡及促进自噬的特性被证实可改善镉诱导的学习记忆障碍、减轻海马区的神经退行性变[126]。因此,未来研究可探索将抗氧化剂与螯合剂联合使用,以期获得协同增效的治疗效果。
2.2.6 铅与神经退行性疾病 铅(Pb)作为世界卫生组织确认的十大危害公众健康的化学物质之一,是一种具有高毒性的重金属污染物[127]。铅暴露在世界各地非常普遍,主要通过肺部吸收含铅气溶胶、胃肠道摄入受铅污染的食物以及皮肤表面吸收含铅灰尘进入血液。铅在人体内呈现显著的生物蓄积性,它的生物半衰期为10-30年,并且可取代骨骼中羟基磷灰石结构中的钙,因此,老年人铅暴露主要来源于骨骼中长期蓄积的内源性铅,随着骨质流失而重新进入血液并变得具有生物活性[128-129]。依据世界卫生组织2021年全球疾病负担研究,铅暴露导致全球约1.2%的伤残调整生命年损失[130],神经系统疾病或障碍占疾病负担的71%,其中包含对儿童智力和行为问题的永久性影响[131-132]。在全球5岁以下儿童中,每年约有7.65亿个智商点因铅暴露而丢失,铅暴露造成的经济损失约为6.0万亿美元[133]。
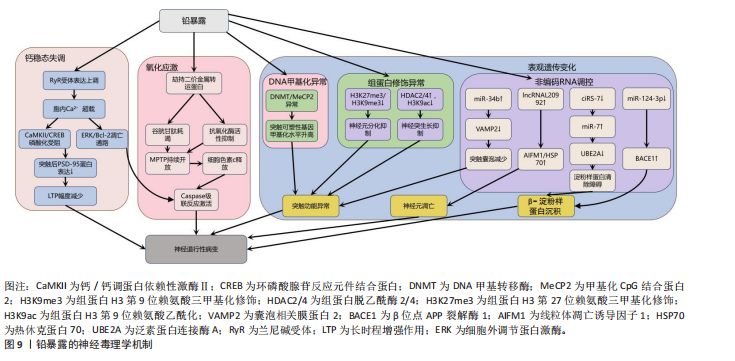
铅暴露与肌萎缩侧索硬化症[134]、阿尔茨海默病[135]、帕金森病等神经退行性疾病的风险增加相关[136]。作为已知的神经毒物,铅通过诱导氧化应激、内质网应激、神经炎症、细胞凋亡、表观遗传变化、兴奋毒性和脑中必需金属的破坏等多种机制引发非特异性脑损伤。
首先,铅是一种氧化还原惰性金属,通过劫持运输铁和铜等必需金属的二价金属转运蛋白进入细胞,消耗含巯基的谷胱甘肽等抗氧化剂并抑制抗氧化关键酶活性,破坏抗氧化防御系统从而引起氧化应激[22,137]。过度的氧化应激诱导线粒体膜通透性转换
孔的持续开放,致使膜电位崩溃和细胞色素c释放,进而激活caspase级联凋亡通路导致神经元凋亡[138]。其次,铅离子(Pb2?)介导兰尼碱受体表达上调,提升神经元细胞内游离钙浓度,会引起钙/钙调蛋白依赖性激酶Ⅱ和环磷酸腺苷的反应元件结合蛋白自磷酸化受阻,并激活钙依赖性细胞外调节蛋白激酶/Bcl-2细胞凋亡信号通路,使突触后致密区PSD-95蛋白表达量下降,最终造成海马区长时程增强幅度减少,可延缓与认知功能相关的神经突生长,与认知功能障碍密切相关[139]。
另外,铅暴露还通过表观遗传机制(包括DNA甲基化、组蛋白修饰和非编码RNA调控)引发神经毒性。在DNA甲基化层面,铅暴露导致DNA甲基转移酶和甲基化CpG结合蛋白2表达异常,影响突触可塑性基因[140-141]。在组蛋白修饰层面,组蛋白H3第9位赖氨酸三甲基化修饰和组蛋白H3第27位赖氨酸三甲基化修饰的减少抑制神经元分化相关基因表达[141],组蛋白脱乙酰酶2/4上调则通过去乙酰化削弱神经突生长和记忆功能[142],而敲除组蛋白脱乙酰酶2可防止铅暴露导致的神经突生长障碍[143]。在非编码RNA层面,
miR-34b基因通过抑制囊泡相关膜蛋白2减少突触囊泡形成[144],铅暴露抑制miR-124-3p基因表达解除其对β位点APP裂解酶1的抑制,加剧β-淀粉样蛋白沉积[145-146]。而在铅暴露的神经毒性调控中,非编码RNA发挥着关键作用,其中长链非编码 RNA与环状 RNA的作用尤为突出。长链非编码RNA内的lncRNA-L20992可调控线粒体凋亡诱导因子1、热休克蛋白70等凋亡蛋白,促使神经元凋亡进而引发神经损伤[147]。低水平的环状RNA7则会导致miR-7表达增加,从而抑制泛素蛋白连接酶A的活性,阻碍阿尔茨海默病患者大脑中β-淀粉样蛋白的正常清除,最终加重神经损伤程度[148]。这些机制共同引发神经元凋亡、炎症及突触功能异常,最终导致认知障碍和神经退行性疾病,见图9[149-153]。
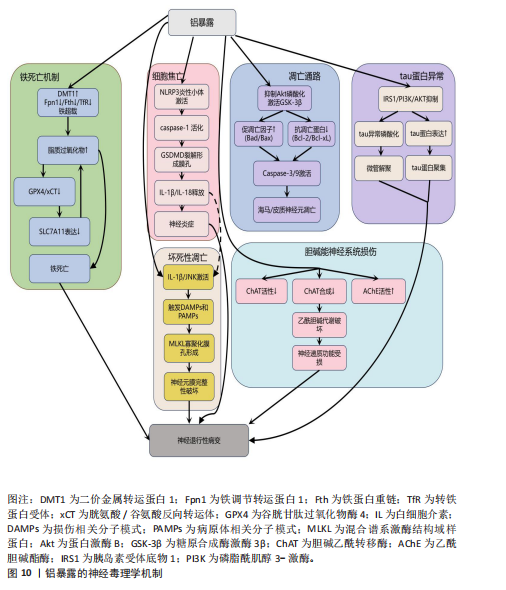
2.2.7 铝与神经退行性疾病 铝(Al)是自然环境中最普遍和最广泛的金属之一,被广泛应用于运输、建筑、军事和家庭应用(厨房用具)[154]。人体主要通过消化道摄入含铝食物和抗酸药物、吸入铝粉尘和皮肤接触含铝化妆品三大途径接触铝元素,其中饮食摄入占总暴露量的95%[155-158]。
铝是众所周知的神经毒素[159],与神经系统疾病的发生密切有关,如自闭症谱系障碍、运动神经元变性、阿尔茨海默病、帕金森病、多发性硬化等[160-161]。铝的神经毒性通过影响神经递质传导[162]、引发氧化应激[163]、诱导神经细胞程序性死亡[164]、损害突触可塑性[165]、导致β-淀粉样蛋白蓄积和tau蛋白异常磷酸化等机制产生[166],具体见图10。
铝暴露通过抑制蛋白激酶B磷酸化解除它对糖原合成酶激酶3β活性的抑制,导致该激酶活性异常升高,这种激酶活性失衡引发双重凋亡效应:一方面,上调促凋亡因子Bad和Bax的表达并激活caspase-3/9级联反应;另一方面,下调抗凋亡蛋白Bcl-2和Bcl-xL水平,这些变化共同导致大鼠海马与皮质神经元发生特异性凋亡,并伴随突触可塑性相关蛋白表达
谱的紊乱,进而引起脑功能连接强度降低,最终表现为学习和记忆功能障碍[167-170]。与此临床前证据相呼应,一项职业流行病学的病例-对照研究发现,职业铝暴露工人的认知障碍与其大脑静息态功能连接的改变密切相关,血浆铝浓度越高,脑功能连接值和认知功能水平越低[171]。
此外,铝还能通过促进白细胞介素1β与其受体结合来激活c-Jun氨基末端激酶信号,这一激活过程触发细胞释放损伤相关分子模式和病原体相关分子模式,这些分子模式被周围吞噬细胞的肿瘤坏死因子受体1或Toll样受体识别后,会募集受体相互作用蛋白形成受体相互作用蛋白1-受体相互作用蛋白3复合物(即坏死小体),坏死小体进而催化混合谱系激酶结构域样蛋白关键位点的磷酸化与泛素化,促使活化的谱系激酶结构域样蛋白寡聚化并转位至细胞膜形成孔道,破坏膜完整性,最终引发坏死性凋
亡[172-174]。
另一重要机制涉及铝暴露激活NLRP3炎性小体介导的细胞焦亡通路。在此通路中,caspase-1被招募到炎性小体的胞质支架上并被激活,活化的caspase-1裂解Gasdermin D,诱导细胞膜孔形成,并释放炎性因子白细胞介素1β和白细胞介素18引发神经炎症,最终导致中枢神经系统损伤[175-176]。研究证实,铝暴露显著上调BV2小胶质细胞焦亡的核心标志物(NLRP3、ASC、cleaved caspase-1、Gasdermin D)及下游炎症因子(白细胞介素1β、白细胞介素18),表明小胶质细胞焦亡是铝诱导神经死亡的潜在机制[177]。进一步研究还发现,纳米氧化铝与慢性应激的协同作用能通过激活组织蛋白酶B/NLRP3轴驱动海马小胶质细胞焦亡,而单独铝暴露无法复现此效应,这提示环境应激因子在铝神经毒性中具有显著的协同增效作用[178]。
铝暴露还通过扰乱铁代谢稳态诱导铁死亡:一方面,铝暴露上调二价金属转运蛋白1促进铁离子摄入;另一方面,它同时抑制铁调节转运蛋白1、铁蛋白重链和转铁蛋白受体表达,显著减少铁的输出与储存能力,这种双向调控的失衡导致细胞内铁离子异常蓄积,形成铁超载状态,过量的游离铁通过芬顿反应与细胞内累积的活性氧协同催化脂质过氧化物生成[179-180]。
与此同时,铝暴露还抑制关键的抗氧化防御系统:它下调胱氨酸/谷氨酸反向转运体和谷胱甘肽过氧化物酶4表达,直接减少谷胱甘肽的合成以及削弱细胞清除脂质过氧化物的能力。铝激活肿瘤抑制因子p53依赖性通路抑制SLC7A11基因表达,进一步阻断了胱氨酸的摄取途径和谷胱甘肽的再生过程,最终因脂质过氧化物不可控的过度积累而触发铁死亡[23,181]。
铝主要通过3种途径破坏乙酰胆碱代谢,破坏胆碱能神经元的功能:抑制胆碱乙酰转移酶活性,破坏胆碱能细胞或减少胆碱乙酰转移酶的合成,增加乙酰胆碱酯酶活性[182-184]。胆碱能神经系统的这种严重损害是导致认知功能障碍、学习记忆能力减退等痴呆样症状的关键因素。
最后,铝暴露还抑制胰岛素受体底物1/磷脂酰肌醇3-激酶/蛋白激酶B通路,该通路的抑制导致tau蛋白表达增加、发生异常磷酸化并聚集,影响其微管组装的正常功能,致使微管解聚。同时,铝启动β-淀粉样蛋白级联反应导致β-淀粉样蛋白1-42沉积[166]。并且,铝还阻碍淀粉样前体蛋白的降解过程,进一步加剧β-淀粉样肽异常积累。铝的这些作用在多个生物模型中均显示出其独特的神经毒性[156]。
抗氧化剂在减轻铝所致神经毒性方面效果显著。例如,强效抗氧化剂褪黑素不仅能减少自由基生成、降低氧化应激标志物(如丙二醛)含量,还能减少铝在大鼠脑中的蓄积,改善铝诱导的记忆与认知障碍[185]。铝螯合剂通过结合体内过量铝降低其毒性,如三价螯合剂去铁胺已被用于治疗铝中毒,并能在一定程度上延缓阿尔茨海默病的进展[186]。此外,铁死亡抑制剂(如利普司他丁1或铁抑素1)可部分减轻β-淀粉样蛋白1–42诱导的神经元死亡;在阿尔茨海默病小鼠模型中,利普司他丁1处理还能改善β-淀粉样蛋白暴露引发的学习记忆障碍[187]。天然化合物如迷迭香酸和香芹酚可通过减少铝蓄积和保护组织免受脂质过氧化损伤来减轻氧化应激[188]。芹菜素可改善AlCl?诱导大鼠神经毒性的抗氧化、神经调节及记忆增强效应[189]。
2.3 金属毒性对神经组织工程的启示
2.3.1 金属污染物对组织工程支架的潜在风险 金属材料凭借优异的机械性能和耐腐蚀性,在生物医学领域特别是组织工程支架方面应用广泛[190-191]。然而,金属植入物在体内环境中有可能释放有毒离子引发炎症、过敏等不良反应,构成了潜在的污染风险[192]。在神经组织工程领域,这种风险尤为值得警惕,因为释放的金属离子可能对敏感的神经细胞产生毒性作用,严重干扰神经再生与修复的关键过程。例如,锌离子虽然是神经组织正常生理功能所必需的微量元素,但过量释放会诱发氧化应激、兴奋性毒性以及线粒体功能障碍,最终导致神经细胞凋亡或坏死。同样,铁离子在脑组织中的异常积累能够催化芬顿反应,产生高活性的羟基自由基加剧氧化应激和炎症反应,最终造成神经细胞死亡。
为了有效降低金属支架带来的潜在风险,研究者们正积极寻求多种策略:一方面,通过优化支架设计,如增加孔隙率以降低模量,使支架更接近天然骨组织的力学特性,同时有助于减少金属离子的释放量[193-195];另一方面,应用表面涂层技术(如银纳米颗粒涂层)在赋予材料抗菌性能的同时,也能有效抑制金属离子溶出[196-198]。
2.3.2 金属螯合剂在神经再生疗法中的协同应用 金属螯合剂的应用为解决神经再生疗法中的金属毒性问题提供了新思路,这类物质能与游离金属离子形成稳定的复合物,显著降低金属离子在体内的浓度和毒性。在神经再生领域,螯合剂展现出与干细胞移植等技术的协同潜力,它们能有效清除微环境中的重金属污染物,为神经再生创造更有利的条件。例如,在阿尔茨海默病研究中,针对铁过载与氧化应激的关联,铁离子螯合治疗已被证实有效[199];螯合剂不仅能溶解阿尔茨海默病患者脑组织中的β-淀粉样蛋白缠结,减少沉积,还能通过抑制自由基生成来减轻神经细胞的氧化损伤[200]。在创伤性脑损伤等情况下,螯合剂可与干细胞移植协同作用:干细胞促进神经修复,而螯合剂则清除损伤部位积累的有害金属离子,降低氧化应激和炎症反应,从而提升移植干细胞的存活率、分化效率及整体神经修复效果[201]。
2.3.3 金属毒性干预的组织工程策略 鉴于金属毒性(尤其是对神经系统的损害)构成严重健康威胁,开发有效的干预策略对神经组织工程至关重要。近年来,该领域在金属毒性干预方面取得显著进展,一个代表性策略是利用搭载螯合剂(如EDTA)的纳米载体进行靶向清除。例如有研究者开发负载乙二胺四乙酸钙的聚合物纳米颗粒,该材料具有良好的生物相容性和靶向性,能穿越血脑屏障精准递送至脑组织,高效螯合铅等重金属离子并促进其排出[202]。实验证明,这种纳米颗粒能显著降低脑内铅含量,减轻神经损伤并改善功能,为重金属中毒治疗提供了新途径[203]。
另一个重要方向是开发能响应金属离子浓度变化的智能生物材料,以调控神经再生并维护神经微环境稳态。环境敏感型水凝胶是近年来发展迅速的一种药物递送新剂型,它能基于不同生理环境在用药局部形成黏附性好的半固体,局部滞留时间长,有利于持续释药[204]。设想未来可以开发一种对金属离子浓度敏感的水凝胶,如Cu2?,当环境中Cu2?浓度升高时(可能指示毒性或失衡),水凝胶的交联程度增加,从而释放出更多生物活性分子以促进神经细胞生长分化;反之,当Cu2?浓度降低时,水凝胶交联度减弱,减少释放,维持再生过程的稳定性。这种智能响应机制对于管理锰、铁、锌、铜等必需但过量积累会引发神经毒性的微量元素尤为重要。
综上所述,理解金属毒性及其机制对神经组织工程具有深远启示。通过优化支架设计、应用螯合剂(尤其是与再生疗法协同)以及发展先进的靶向递送系统和智能响应材料,能够有效降低金属毒性风险,清除有害金属,并精细调控神经微环境。这些策略不仅为减轻金属对神经系统的损害提供了有力工具,更开辟了促进神经再生与修复的新路径。未来研究的重点在于优化这些策略的安全性、效率和临床转化潜力,以推动神经组织工程迈向更可靠、更有效的临床应用。

| [1] NIKOM D, ZHENG S. Alternative splicing in neurodegenerative disease and the promise of RNA therapies. Nat Rev Neurosci. 2023; 24(8):457-473. [2] LI D, ZHOU L, CAO Z, et al. Associations of environmental factors with neurodegeneration: An exposome-wide Mendelian randomization investigation. Ageing Res Rev. 2024;95:102254. [3] CIUREA AV, MOHAN AG, COVACHE-BUSUIOC RA, et al. Unraveling Molecular and Genetic Insights into Neurodegenerative Diseases: Advances in Understanding Alzheimer’s, Parkinson’s, and Huntington’s Diseases and Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2023;24(13):10809. [4] SUN J, ROY S. Gene-based therapies for neurodegenerative diseases. Nat Neurosci. 2021;24(3):297-311. [5] HESTER K, KIRRANE E, ANDERSON T, et al. Environmental exposure to metals and the development of tauopathies, synucleinopathies, and TDP-43 proteinopathies: A systematic evidence map protocol. Environ Int. 2022;169: 107528. [6] PANDICS T, MAJOR D, FAZEKAS-PONGOR V, et al. Exposome and unhealthy aging: environmental drivers from air pollution to occupational exposures. GeroScience. 2023;45(6):3381-3408. [7] WILSON DM, COOKSON MR, VAN DEN BOSCH L, et al. Hallmarks of neurodegenerative diseases. Cell. 2023; 186(4):693-714. [8] VERMEULEN R, SCHYMANSKI EL, BARABÁSI AL, et al. The exposome and health: Where chemistry meets biology. Science. 2020;367(6476):392-396. [9] SONG Q, LI J. Environmental effects of heavy metals derived from the e-waste recycling activities in China: A systematic review. WASTE Manag. 2014;34(12):2587-2594. [10] CHU Z, FAN X, WANG W, et al. Quantitative evaluation of heavy metals’ pollution hazards and estimation of heavy metals’ environmental costs in leachate during food waste composting. WASTE Manag. 2019;84:119-128. [11] COSTA LG, COLE TB, DAO K, et al. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol Ther. 2020;210:107523. [12] CHEN YG, HE XLS, HUANG JH, et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol Environ Saf. 2021;219:112336. [13] SU Z, LI X, XI Y, et al. Microbe-mediated transformation of metal sulfides: Mechanisms and environmental significance. Sci TOTAL Environ. 2022;825: 153767. [14] MAJUMDAR A, UPADHYAY MK, OJHA M, et al. A critical review on the organo-metal(loid)s pollution in the environment: Distribution, remediation and risk assessment. Sci Total Environ. 2024;951:175531. [15] SUN Y, LEI S, ZHAO Y, et al. Spatial distribution prediction of soil heavy metals based on sparse sampling and multi-source environmental data. J Hazard Mater. 2024;465:133114. [16] ZHANG X, ZHANG P, WEI X, et al. Migration, transformation of arsenic, and pollution controlling strategies in paddy soil-rice system: A comprehensive review. Sci Total Environ. 2024;951:175500. [17] HARISCHANDRA DS, GHAISAS S, ZENITSKY G, et al. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front Neurosci. 2019;13:654. [18] ZENG T, LI J, XIE L, et al. Nrf2 regulates iron-dependent hippocampal synapses and functional connectivity damage in depression. J Neuroinflammation. 2023; 20(1):212. [19] LIPPI SLP, SMITH ML, FLINN JM. A Novel hAPP/htau Mouse Model of Alzheimer’s Disease: Inclusion of APP With Tau Exacerbates Behavioral Deficits and Zinc Administration Heightens Tangle Pathology. Front Aging Neurosci. 2018;10:382. [20] PATEL R, ASCHNER M. Commonalities between Copper Neurotoxicity and Alzheimer’s Disease. Toxics. 2021;9(1):4. [21] DENG P, FAN T, GAO P, et al. SIRT5-Mediated Desuccinylation of RAB7A Protects Against Cadmium-Induced Alzheimer’s Disease-Like Pathology by Restoring Autophagic Flux. Adv Sci Weinh Baden-Wurtt Ger. 2024; 11(30):e2402030. [22] BALALI-MOOD M, NASERI K, TAHERGORABI Z, et al. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front Pharmacol. 2021;12:643972. [23] ZHANG H, JIAO W, CUI H, et al. Combined exposure of alumina nanoparticles and chronic stress exacerbates hippocampal neuronal ferroptosis via activating IFN-γ/ASK1/JNK signaling pathway in rats. J Hazard Mater. 2021;411:125179. [24] WILD CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2005; 14(8):1847-1850. [25] STEWART WF, SCHWARTZ BS, DAVATZIKOS C, et al. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66(10):1476-1484. [26] WU J, BASHA MR, BROCK B, et al. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci Off J Soc Neurosci. 2008;28(1):3-9. [27] GU H, WEI X, MONNOT AD, et al. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci Lett. 2011;490(1):16-20. [28] ASHOK A, RAI NK, TRIPATHI S, et al. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci Off J Soc Toxicol. 2015;143(1):64-80. [29] GENOUD S, SENIOR AM, HARE DJ, et al. Meta-Analysis of Copper and Iron in Parkinson’s Disease Brain and Biofluids. Mov Disord Off J Mov Disord Soc. 2020; 35(4):662-671. [30] DOROSZKIEWICZ J, FARHAN J A, MROCZKO J, et al. Common and Trace Metals in Alzheimer’s and Parkinson’s Diseases. Int J Mol Sci. 2023;24(21):15721. [31] LIVINGSTON G, HUNTLEY J, LIU KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet Lond Engl. 2024;404(10452):572-628. [32] LU L, ZHANG Y, ANGLEY M, et al. Association of Urinary Cadmium Concentration With Cognitive Impairment in US Adults: A Longitudinal Cohort Study. Neurology. 2024; 103(7):e209808. [33] KIM H, HARRISON FE, ASCHNER M, et al. Exposing the role of metals in neurological disorders: a focus on manganese. Trends Mol Med. 2022;28(7):555-568. [34] HORNING KJ, CAITO SW, TIPPS KG, et al. Manganese Is Essential for Neuronal Health. Annu Rev Nutr. 2015;35:71-108. [35] LV M, CHEN M, ZHANG R, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020; 30(11):966-979. [36] MARTINS AC, GUBERT P, VILLAS BOAS GR, et al. Manganese-induced neurodegenerative diseases and possible therapeutic approaches. Expert Rev Neurother. 2020;20(11):1109-1121. [37] BUDINGER D, BARRAL S, SOO AKS, et al. The role of manganese dysregulation in neurological disease: emerging evidence. Lancet Neurol. 2021;20(11):956-968. [38] KOSUTH T, LESKOVA A, CASTAINGS L, et al. Golgi in and out: multifaceted role and journey of manganese. New Phytol. 2023; 238(5):1795-1800. [39] BALACHANDRAN RC, MUKHOPADHYAY S, MCBRIDE D, et al. Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem. 2020;295(19): 6312-6329. [40] CRISWELL SR, NIELSEN SS, WARDEN MN, et al. MRI Signal Intensity and Parkinsonism in Manganese-Exposed Workers. J Occup Environ Med. 2019;61(8):641-645. [41] LEE EY, FLYNN MR, DU G, et al. Nigral MRI features of asymptomatic welders. Parkinsonism Relat Disord. 2021;85:37-43. [42] XU B, HUANG S, LIU Y, et al. Manganese promotes α-synuclein amyloid aggregation through the induction of protein phase transition. J Biol Chem. 2022;298(1):101469. [43] BAJ J, FLIEGER W, BARBACHOWSKA A, et al. Consequences of Disturbing Manganese Homeostasis. Int J Mol Sci. 2023;24(19): 14959. [44] KIM SG, CHOE YM, SUH GH, et al. Manganese level and cognitive decline in older adults with the APOE e4 allele: a preliminary study. Psychiatry Res. 2023; 327:115403. [45] PRADHAN SH, LIU JY, SAYES CM. Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson’s Disease Model. Int J Mol Sci. 2023;24(22):16129. [46] Smith MR, Fernandes J, Go YM, et al. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem Biophys Res Commun. 2017;482(3):388-398. [47] WARREN EB, BRYAN MR, MORCILLO P, et al. Manganese-induced Mitochondrial Dysfunction Is Not Detectable at Exposures Below the Acute Cytotoxic Threshold in Neuronal Cell Types. Toxicol Sci Off J Soc Toxicol. 2020;176(2):446-459. [48] PIRUNKASET E, BOONYARAT C, MANEENET J, et al. Effect of Diacetylcurcumin Manganese Complex on Rotenone-Induced Oxidative Stress, Mitochondria Dysfunction, and Inflammation in the SH-SY5Y Parkinson’s Disease Cell Model. Molecules. 2024; 29(5):957. [49] ZHU G, LIU Y, ZHI Y, et al. PKA- and Ca2+-dependent p38 MAPK/CREB activation protects against manganese-mediated neuronal apoptosis. Toxicol Lett. 2019;309: 10-19. [50] HARISCHANDRA DS, JIN H, ANANTHARAM V, et al. α-Synuclein protects against manganese neurotoxic insult during the early stages of exposure in a dopaminergic cell model of Parkinson’s disease. Toxicol Sci Off J Soc Toxicol. 2015;143(2):454-468. [51] CHIB S, SINGH S. Manganese and related neurotoxic pathways: A potential therapeutic target in neurodegenerative diseases. Neurotoxicol Teratol. 2022;94: 107124. [52] KIRKLEY KS, POPICHAK KA, AFZALI MF, et al. Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J Neuroinflammation. 2017;14(1):99. [53] KE T, SIDORYK-WEGRZYNOWICZ M, PAJARILLO E, et al. Role of Astrocytes in Manganese Neurotoxicity Revisited. Neurochem Res. 2019;44(11):2449-2459. [54] POPICHAK KA, AFZALI MF, KIRKLEY KS, et al. Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese. J Neuroinflammation. 2018; 15(1):324. [55] BRYAN MR, BOWMAN AB. Manganese and the Insulin-IGF Signaling Network in Huntington’s Disease and Other Neurodegenerative Disorders. Adv Neurobiol. 2017;18:113-142. [56] HARISCHANDRA DS, ROKAD D, NEAL ML, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Sci Signal. 2019;12(572):eaau4543. [57] PAJARILLO E, NYARKO-DANQUAH I, DIGMAN A, et al. Mechanisms of manganese-induced neurotoxicity and the pursuit of neurotherapeutic strategies. Front Pharmacol. 2022;13:1011947. [58] SARKAR S, MALOVIC E, HARISCHANDRA DS, et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology. 2018;64:204-218. [59] QIAN K, JIANG X, LIU ZQ, et al. Revisiting the critical roles of reactive astrocytes in neurodegeneration. Mol Psychiatry. 2023;28(7):2697-2706. [60] SINGH S, SHAIKH IA, MORE SS, et al. Blockage of KHSRP-NLRP3 by MCC950 Can Reverse the Effect of Manganese-Induced Neuroinflammation in N2a Cells and Rat Brain. Int J Mol Sci. 2022;23(21):13224. [61] TARNACKA B, JOPOWICZ A, MAŚLIŃSKA M. Copper, Iron, and Manganese Toxicity in Neuropsychiatric Conditions. Int J Mol Sci. 2021;22(15):7820. [62] KAWAHARA M, KATO-NEGISHI M, TANAKA KI. Dietary Trace Elements and the Pathogenesis of Neurodegenerative Diseases. Nutrients. 2023;15(9):2067. [63] FRYDRYCH A, KROŚNIAK M, JUROWSKI K. The Role of Chosen Essential Elements (Zn, Cu, Se, Fe, Mn) in Food for Special Medical Purposes (FSMPs) Dedicated to Oncology Patients-Critical Review: State-of-the-Art. Nutrients. 2023;15(4):1012. [64] GENG H, LI Z, LI Z, et al. Restoring neuronal iron homeostasis revitalizes neurogenesis after spinal cord injury. Proc Natl Acad Sci U S A. 2023;120(46):e2220300120. [65] XIAO G, ZHAO M, LIU Z, et al. Zinc antagonizes iron-regulation of tyrosine hydroxylase activity and dopamine production in Drosophila melanogaster. BMC Biol. 2021;19(1):236. [66] HALCROW PW, LYNCH ML, GEIGER JD, et al. Role of endolysosome function in iron metabolism and brain carcinogenesis. Semin Cancer Biol. 2021;76:74-85. [67] YANG WS, STOCKWELL BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26(3):165-176. [68] DING XS, GAO L, HAN Z, et al. Ferroptosis in Parkinson’s disease: Molecular mechanisms and therapeutic potential. Ageing Res Rev. 2023;91:102077. [69] MAHONEY-SÁNCHEZ L, BOUCHAOUI H, AYTON S, et al. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog Neurobiol. 2021;196:101890. [70] LAN T, SUN TT, WEI C, et al. Epigenetic Regulation of Ferroptosis in Central Nervous System Diseases. Mol Neurobiol. 2023;60(7):3584-3599. [71] WU M, CHEN Z, JIANG M, et al. Friend or foe: role of pathological tau in neuronal death. Mol Psychiatry. 2023;28(6):2215-2227. [72] MA J, GUO Q, SHEN MQ, et al. Apolipoprotein E is required for brain iron homeostasis in mice. Redox Biol. 2023;64:102779. [73] LI XN, SHANG NY, KANG YY, et al. Caffeic acid alleviates cerebral ischemic injury in rats by resisting ferroptosis via Nrf2 signaling pathway. Acta Pharmacol Sin. 2024;45(2):248-267. [74] WARD RJ, DEXTER DT, CRICHTON RR. Iron, Neuroinflammation and Neurodegeneration. Int J Mol Sci. 2022; 23(13):7267. [75] WANG Y, LV MN, ZHAO WJ. Research on ferroptosis as a therapeutic target for the treatment of neurodegenerative diseases. Ageing Res Rev. 2023;91:102035. [76] BAYAZID AB, LIM BO. Quercetin Is An Active Agent in Berries against Neurodegenerative Diseases Progression through Modulation of Nrf2/HO1. Nutrients. 2022;14(23):5132. [77] KUMAR V, KUMAR A, SINGH K, et al. Neurobiology of zinc and its role in neurogenesis. Eur J Nutr. 2021;60(1):55-64. [78] LI Z, LIU Y, WEI R, et al. The Important Role of Zinc in Neurological Diseases. Biomolecules. 2022;13(1):28. [79] MIZUNO D, KAWAHARA M. The molecular mechanisms of zinc neurotoxicity and the pathogenesis of vascular type senile dementia. Int J Mol Sci. 2013;14(11):22067-22081. [80] CHOI S, HONG DK, CHOI BY, et al. Zinc in the Brain: Friend or Foe? Int J Mol Sci. 2020; 21(23): 8941. [81] TÓTH K. Zinc in neurotransmission. Annu Rev Nutr. 2011;31:139-153. [82] WANG J, DENG X, ZHANG F, et al. ZnO nanoparticle-induced oxidative stress triggers apoptosis by activating JNK signaling pathway in cultured primary astrocytes. Nanoscale Res Lett. 2014;9(1):117. [83] SUN R, WANG J, FENG J, et al. Zinc in Cognitive Impairment and Aging. Biomolecules. 2022; 12(7):1000. [84] SENSI SL, GRANZOTTO A, SIOTTO M, et al. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharmacol Sci. 2018;39(12):1049-1063. [85] GROMADZKA G, TARNACKA B, FLAGA A, et al. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int J Mol Sci. 2020;21(23):9259. [86] RODRIGUEZ P, KALIA V, FENOLLAR-FERRER C, et al. Glial swip-10 controls systemic mitochondrial function, oxidative stress, and neuronal viability via copper ion homeostasis. Proc Natl Acad Sci U S A. 2024;121(39):e2320611121. [87] JOMOVA K, CVIK M, LAURO P, et al. The role of redox active copper(II) on antioxidant properties of the flavonoid baicalein: DNA protection under Cu(II)-Fenton reaction and Cu(II)-ascorbate system conditions. J Inorg Biochem. 2023;245:112244. [88] SIMUNKOVA M, BARBIERIKOVA Z, JOMOVA K, et al. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int J Mol Sci. 2021; 22(4):1619. [89] KOLA A, VIGNI G, LAMPONI S, et al. Protective Contribution of Rosmarinic Acid in Rosemary Extract Against Copper-Induced Oxidative Stress. Antioxid Basel Switz. 2024;13(11):1419. [90] ZHONG G, WANG X, LI J, et al. Insights Into the Role of Copper in Neurodegenerative Diseases and the Therapeutic Potential of Natural Compounds. Curr Neuropharmacol. 2024;22(10):1650-1671. [91] HUANG M, ZHANG Y, LIU X. The mechanism of cuproptosis in Parkinson’s disease. Ageing Res Rev. 2024;95:102214. [92] OKAFOR M, GONZALEZ P, RONOT P, et al. Development of Cu(ii)-specific peptide shuttles capable of preventing Cu-amyloid beta toxicity and importing bioavailable Cu into cells. Chem Sci. 2022;13(40):11829-11840. [93] SINGH SK, BALENDRA V, OBAID AA, et al. Copper-mediated β-amyloid toxicity and its chelation therapy in Alzheimer’s disease. Met Integr Biometal Sci. 2022; 14(6):mfac018. [94] WITTUNG-STAFSHEDE P. Crossroads between copper ions and amyloid formation in Parkinson’s disease. Essays Biochem. 2022;66(7):977-986. [95] BACCHELLA C, CAMPONESCHI F, KOLKOWSKA P, et al. Copper Binding and Redox Activity of α-Synuclein in Membrane-Like Environment. Biomolecules. 2023; 13(2):287. [96] VARHAUG KN, KRÅKENES T, ALME MN, et al. Mitochondrial complex IV is lost in neurons in the cuprizone mouse model. Mitochondrion. 2020;50:58-62. [97] RUIZ LM, LIBEDINSKY A, ELORZA AA. Role of Copper on Mitochondrial Function and Metabolism. Front Mol Biosci. 2021;8: 711227. [98] CHEN L, MIN J, WANG F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7(1):378. [99] CRUCES-SANDE A, RODRÍGUEZ-PÉREZ AI, HERBELLO-HERMELO P, et al. Copper Increases Brain Oxidative Stress and Enhances the Ability of 6-Hydroxydopamine to Cause Dopaminergic Degeneration in a Rat Model of Parkinson’s Disease. Mol Neurobiol. 2019;56(4):2845-2854. [100] SCHMIDT K, RALLE M, SCHAFFER T, et al. ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine-β-hydroxylase. J Biol Chem. 2018;293(52):20085-20098. [101] SANDE R, DOSHI G, GODAD A. Deciphering the role of metal and non-metals in the treatment of epilepsy. Neurochem Int. 2023;167:105536. [102] D’AMBROSI N, ROSSI L. Copper at synapse: Release, binding and modulation of neurotransmission. Neurochem Int. 2015; 90:36-45. [103] RASCHKE S, BORNHORST J, SCHWERDTLE T. Se supplementation to an in vitro blood-brain barrier does not affect Cu transfer into the brain. J Trace Elem Med Biol. 2023; 78:127180. [104] BISAGLIA M, BUBACCO L. Copper Ions and Parkinson’s Disease: Why Is Homeostasis So Relevant? Biomolecules. 2020;10(2):195. [105] WANG Y, LI D, XU K, et al. Copper homeostasis and neurodegenerative diseases. Neural Regen Res. 2025;20(11): 3124-3143. [106] FILIPPINI T, TORRES D, LOPES C, et al.Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ Int. 2020;142: 105879. [107] STRAIF K, BENBRAHIM-TALLAA L, BAAN R, et al. A review of human carcinogens--Part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10(5):453-454. [108] WANG R, SANG P, GUO Y, et al. Cadmium in food: Source, distribution and removal. Food Chem. 2023;405(Pt A):134666. [109] REZAEI K, MASTALI G, ABBASGHOLINEJAD E, et al. Cadmium neurotoxicity: Insights into behavioral effect and neurodegenerative diseases. Chemosphere. 2024;364:143180. [110] OGGIANO R, PISANO A, SABALIC A, et al.An overview on amyotrophic lateral sclerosis and cadmium. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021;42(2):531-537. [111] MA Y, SU Q, YUE C, et al. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int J Mol Sci. 2022;23(21):13491. [112] JOMOVA K, ALOMAR SY, NEPOVIMOVA E, et al. Heavy metals: toxicity and human health effects. Arch Toxicol. 2025, 99(1):153-209. [113] CAO X, FU M, BI R, et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere. 2021;263:128346. [114] PARK JH, LEE BM, KIM HS. Potential protective roles of curcumin against cadmium-induced toxicity and oxidative stress. J Toxicol Environ Health B Crit Rev. 2021;24(3):95-118. [115] CARRASCO J, GIRALT M, MOLINERO A, et al. Metallothionein (MT)-III: generation of polyclonal antibodies, comparison with MT-I+II in the freeze lesioned rat brain and in a bioassay with astrocytes, and analysis of Alzheimer’s disease brains. J Neurotrauma. 1999;16(11):1115-1129. [116] NAVARRO-SEMPERE A, MARTÍNEZ-PEINADO P, RODRIGUES AS, et al. Metallothionein expression in the central nervous system in response to chronic heavy metal exposure: possible neuroprotective mechanism. Environ Geochem Health. 2023;45(11):8257-8269. [117] VAŠÁK M, MELONI G. Mammalian Metallothionein-3: New Functional and Structural Insights. Int J Mol Sci. 2017; 18(6):1117. [118] ARRUEBARRENA MA, HAWE CT, LEE YM, et al. Mechanisms of Cadmium Neurotoxicity. Int J Mol Sci. 2023;24(23): 16558. [119] XU B, CHEN S, LUO Y, et al. Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS One. 2011;6(4):e19052. [120] CHEN S, XU Y, XU B, et al. CaMKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J Neurochem. 2011;119(5):1108-1118. [121] ZHANG T, SUN S, GAVRILOVIĆ A, et al. Selenium alleviates cadmium-induced oxidative stress, endoplasmic reticulum stress, and apoptosis in L8824 cells. Ecotoxicol Environ Saf. 2023;262:115337. [122] GADE M, COMFORT N, RE DB. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ Res. 2021;201:111558. [123] BRANCA JJV, MARESCA M, MORUCCI G, et al. Effects of Cadmium on ZO-1 Tight Junction Integrity of the Blood Brain Barrier. Int J Mol Sci. 2019;20(23):6010. [124] LI CX, TALUKDER M, XU YR, et al. Cadmium aggravates the blood-brain barrier disruption via inhibition of the Wnt7A/β-catenin signaling axis. Environ Pollut. 2023;324:121400. [125] FERRERO ME. Neuron Protection by EDTA May Explain the Successful Outcomes of Toxic Metal Chelation Therapy in Neurodegenerative Diseases. Biomedicines. 2022;10(10):2476. [126] ARAB HH, EID AH, ALSUFYANI SE, et al. Neuroprotective Impact of Linagliptin against Cadmium-Induced Cognitive Impairment and Neuropathological Aberrations: Targeting SIRT1/Nrf2 Axis, Apoptosis, and Autophagy. Pharm Basel Switz. 2023;16(8):1065. [127] MOHAMMED TA, MEIER CM, KALVODA T, et al. Potent Cyclic Tetrapeptide for Lead Detoxification. Angew Chem Int Ed Engl. 2021;60(22):12381-12385. [128] SWARINGEN BF, GAWLIK E, KAMENOV GD, et al. Children’s exposure to environmental lead: A review of potential sources, blood levels, and methods used to reduce exposure. Environ Res. 2022;204(Pt B): 112025. [129] HU H, RABINOWITZ M, SMITH D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998; 106(1):1-8. [130] GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Lond Engl. 2024;403(10440):2162-2203. [131] GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23(4):344-381. [132] SHAFFER RM, SELLERS SP, BAKER MG, et al.Improving and Expanding Estimates of the Global Burden of Disease Due to Environmental Health Risk Factors. Environ Health Perspect. 2019;127(10):105001. [133] LARSEN B, SÁNCHEZ-TRIANA E. Global health burden and cost of lead exposure in children and adults: a health impact and economic modelling analysis. Lancet Planet Health. 2023;7(10):e831-e840. [134] ANDREW A, ZHOU J, GUI J, et al. Airborne lead and polychlorinated biphenyls (PCBs) are associated with amyotrophic lateral sclerosis (ALS) risk in the U.S. Sci Total Environ. 2022;819:153096. [135] BIHAQI SW, BAHMANI A, SUBAIEA GM, et al. Infantile exposure to lead and late-age cognitive decline: relevance to AD. Alzheimers Dement J Alzheimers Assoc. 2014;10(2):187-195. [136] WEISSKOPF MG, WEUVE J, NIE H, et al. Association of cumulative lead exposure with Parkinson’s disease. Environ Health Perspect. 2010;118(11):1609-1613. [137] KATABA A, BOTHA TL, NAKAYAMA SMM, et al. Acute exposure to environmentally relevant lead levels induces oxidative stress and neurobehavioral alterations in larval zebrafish (Danio rerio). Aquat Toxicol Amst Neth. 2020;227:105607. [138] BAI R, GUO J, YE XY, et al. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev. 2022;77:101619. [139] ZHOU F, DU G, XIE J, et al. RyRs mediate lead-induced neurodegenerative disorders through calcium signaling pathways. Sci Total Environ. 2020;701:134901. [140] WANG R, YANG M, WU Y, et al. SIRT1 modifies DNA methylation linked to synaptic deficits induced by Pb in vitro and in vivo. Int J Biol Macromol. 2022;217:219-228. [141] LIN LF, XIE J, SÁNCHEZ OF, et al. Low dose lead exposure induces alterations on heterochromatin hallmarks persisting through SH-SY5Y cell differentiation. Chemosphere. 2021;264(Pt 1):128486. [142] GU X, XU Y, XUE W Z, et al. Interplay of miR-137 and EZH2 contributes to the genome-wide redistribution of H3K27me3 underlying the Pb-induced memory impairment. Cell Death Dis. 2019;10(9):671. [143] ZHOU R, ZHAO J, LI D, et al. Combined exposure of lead and cadmium leads to the aggravated neurotoxicity through regulating the expression of histone deacetylase 2. Chemosphere. 2020;252:126589. [144] YANG C, KANG B, CAO Z, et al. Early-Life Pb Exposure Might Exert Synapse-Toxic Effects Via Inhibiting Synapse-Associated Membrane Protein 2 (VAMP2) Mediated by Upregulation of miR-34b. J Alzheimers Dis. 2022;87(2):619-633. [145] WANG R, WU Z, LIU R, et al. Age-related miRNAs dysregulation and abnormal BACE1 expression following Pb exposure in adolescent mice. Environ Toxicol. 2022; 37(8):1902-1913. [146] SINGH N, DAS B, ZHOU J, et al. Targeted BACE-1 inhibition in microglia enhances amyloid clearance and improved cognitive performance. Sci Adv. 2022;8(29): eabo3610. [147] NAN A, JIA Y, LI X, et al. Editor’s Highlight: lncRNAL20992 Regulates Apoptotic Proteins to Promote Lead-Induced Neuronal Apoptosis. Toxicol Sci Off J Soc Toxicol. 2018;161(1):115-124. [148] KONDO MA, MOHAN A, MATHER KA. Going around in circles: deciphering the role of circular RNAs in neurodegenerative disease. Curr Opin Psychiatry. 2020;33(2):141-147. [149] EID A, BIHAQI SW, RENEHAN WE, et al. Developmental lead exposure and lifespan alterations in epigenetic regulators and their correspondence to biomarkers of Alzheimer’s disease. Alzheimers Dement Amst Neth. 2016;2:123-131. [150] MITRA P, SHARMA S, PUROHIT P, et al. Clinical and molecular aspects of lead toxicity: An update. Crit Rev Clin Lab Sci. 2017;54(7-8):506-528. [151] GUNDACKER C, FORSTHUBER M, SZIGETI T, et al. Lead (Pb) and neurodevelopment: A review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int J Hyg Environ Health. 2021;238:113855. [152] MEI Z, LIU G, ZHAO B, et al. Emerging roles of epigenetics in lead-induced neurotoxicity. Environ Int. 2023;181:108253. [153] MISTRETTA M, FARINI A, TORRENTE Y, et al. Multifaceted nanoparticles: emerging mechanisms and therapies in neurodegenerative diseases. Brain J Neurol. 2023;146(6):2227-2240. [154] WANG C, LI S, GUO Y, et al. Comprehensive treatments of aluminum dross in China: A critical review. J Environ Manage. 2023; 345:118575. [155] BRYLIŃSKI Ł, KOSTELECKA K, WOLIŃSKI F, et al. Aluminium in the Human Brain: Routes of Penetration, Toxicity, and Resulting Complications. Int J Mol Sci. 2023; 24(8):7228. [156] KLOTZ K, WEISTENHÖFER W, NEFF F, et al. The Health Effects of Aluminum Exposure. Dtsch Arzteblatt Int. 2017;114(39):653-659. [157] HILLER J, GÖEN T, SEIBOLD-WULF N, et al. Effect of an aluminum foil-processed diet on internal human aluminum burden. Environ Int. 2023;177:108000. [158] RENKE G, ALMEIDA VBP, SOUZA EA, et al.Clinical Outcomes of the Deleterious Effects of Aluminum on Neuro-Cognition, Inflammation, and Health: A Review. Nutrients. 2023;15(9):2221. [159] YANG L, CHEN L, LI W, et al. METTL3-mediated m6A RNA methylation was involved in aluminum-induced neurotoxicity. Ecotoxicol Environ Saf. 2024;270:115878. [160] SHIANI A, SHARAFI K, OMER AK, et al. A systematic literature review on the association between exposures to toxic elements and an autism spectrum disorder. Sci Total Environ. 2023;857(Pt 2):159246. [161] BRYLIŃSKI Ł, KOSTELECKA K, WOLIŃSKI F, et al. Aluminium in the Human Brain: Routes of Penetration, Toxicity, and Resulting Complications. Int J Mol Sci. 2023;24(8):7228. [162] POHANKA M. Copper, aluminum, iron and calcium inhibit human acetylcholinesterase in vitro. Environ Toxicol Pharmacol. 2014; 37(1):455-459. [163] FERNANDES RM, CORRÊA MG, ARAGÃO WAB, et al. Preclinical evidences of aluminum-induced neurotoxicity in hippocampus and pre-frontal cortex of rats exposed to low doses. Ecotoxicol Environ Saf. 2020;206:111139. [164] MOUJALLED D, STRASSER A, LIDDELL JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28(7):2029-2044. [165] LI H, XUE X, LI L, et al. Aluminum-Induced Synaptic Plasticity Impairment via PI3K-Akt-mTOR Signaling Pathway. Neurotox Res. 2020;37(4):996-1008. [166] HE C, ZHAO X, LEI Y, et al. Whole-transcriptome analysis of aluminum-exposed rat hippocampus and identification of ceRNA networks to investigate neurotoxicity of Al. Mol Ther Nucleic Acids. 2021;26:1401-1417. [167] BITTENCOURT LO, DAMASCENO-SILVA RD, ARAGÃO WAB, et al. Global Proteomic Profile of Aluminum-Induced Hippocampal Impairments in Rats: Are Low Doses of Aluminum Really Safe? Int J Mol Sci. 2022; 23(20):12523. [168] MIU AC, BENGA O. Aluminum and Alzheimer’s disease: a new look. J Alzheimers Dis. 2006;10(2-3):179-201. [169] SHANG N, ZHANG P, WANG S, et al. Aluminum-Induced Cognitive Impairment and PI3K/Akt/mTOR Signaling Pathway Involvement in Occupational Aluminum Workers. Neurotox Res. 2020;38(2): 344-358. [170] YANG X, DU W, ZHANG Y, et al. Neuroprotective Effects of Higenamine Against the Alzheimer’s Disease Via Amelioration of Cognitive Impairment, Aβ Burden, Apoptosis and Regulation of Akt/GSK3β Signaling Pathway. Dose-Response Publ Int Hormesis Soc. 2020;18(4): 1559325820972205. [171] 赵丹,高丹,宦佳萍,等.脑功能连接在职业铝暴露致工人认知功能下降中的中介作用[J].环境与职业医学,2023, 40(3):239-245. [172] GARCIA LR, TENEV T, NEWMAN R, et al.Ubiquitylation of MLKL at lysine 219 positively regulates necroptosis-induced tissue injury and pathogen clearance. Nat Commun. 2021;12(1):3364. [173] BEDOUI S, HEROLD MJ, STRASSER A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020; 21(11):678-695. [174] ZHANG H, WEI M, LU X, et al. Aluminum trichloride caused hippocampal neural cells death and subsequent depression-like behavior in rats via the activation of IL-1β/JNK signaling pathway. Sci Total Environ. 2020;715:136942. [175] XUE W, CUI D, QIU Y. Research Progress of Pyroptosis in Alzheimer’s Disease. Front Mol Neurosci. 2022;15:872471. [176] HAO W, ZHU X, LIU Z, et al. Aluminum exposure induces central nervous system impairment via activating NLRP3-medicated pyroptosis pathway. Ecotoxicol Environ Saf. 2023;264:115401. [177] HAO W, HAO C, WU C, et al. Aluminum impairs cognitive function by activating DDX3X-NLRP3-mediated pyroptosis signaling pathway. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2021;157:112591. [178] ZHANG H, WANG J, RUAN C, et al. Co-exposure of chronic stress and alumina nanoparticles aggravates hippocampal microglia pyroptosis by activating cathepsin B/NLRP3 signaling pathway. J Hazard Mater. 2022;436:129093. [179] ZHANG J, HUANG W, XU F, et al. Iron Dyshomeostasis Participated in Rat Hippocampus Toxicity Caused by Aluminum Chloride. Biol Trace Elem Res. 2020;197(2): 580-590. [180] CHEN K, JIANG X, WU M, et al. Ferroptosis, a Potential Therapeutic Target in Alzheimer’s Disease. Front Cell Dev Biol. 2021;9:704298. [181] CHENG L, LIANG R, LI Z, et al. Aluminum maltolate triggers ferroptosis in neurons: mechanism of action. Toxicol Mech Methods. 2021;31(1):33-42. [182] SZUTOWICZ A. Aluminum, NO, and nerve growth factor neurotoxicity in cholinergic neurons. J Neurosci Res. 2001;66(5):1009-1018. [183] 夏佳蕊,刘佳琪,李宗高,等.铝神经毒性作用机制研究进展[J].中国老年学杂志,2018,38(13): 3276-3280. [184] ABD ELMONEM HA, MORSI RM, MANSOUR DS, et al. Myco-fabricated ZnO nanoparticles ameliorate neurotoxicity in mice model of Alzheimer’s disease via acetylcholinesterase inhibition and oxidative stress reduction. Biometals Int J Role Met Ions Biol Biochem Med. 2023;36(6):1391-1404. [185] PROMYO K, IQBAL F, CHAIDEE N, et al. Aluminum chloride-induced amyloid β accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2020;146:111829. [186] FULGENZI A, VIETTI D, FERRERO ME. Aluminium involvement in neurotoxicity. BioMed Res Int. 2014;2014:758323. [187] BAO WD, PANG P, ZHOU XT, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28(5):1548-1562. [188] BARANAUSKAITE J, SADAUSKIENE I, LIEKIS A, et al. Natural Compounds Rosmarinic Acid and Carvacrol Counteract Aluminium-Induced Oxidative Stress. Mol Basel Switz. 2020;25(8):1807. [189] OYAGBEMI AA, FEMI-AKINLOSOTU OM, OBASA AA, et al. Apigenin mitigates oxidative stress, neuroinflammation, and cognitive impairment but enhances learning and memory in aluminum chloride-induced neurotoxicity in rats. Alzheimers Dement J Alzheimers Assoc. 2025;21(5):e70223. [190] BOBBERT FSL, ZADPOOR AA. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J Mater Chem B. 2017;5(31):6175-6192. [191] COSTA MM, BARTOLOMEU F, ALVES N, et al. Tribological behavior of bioactive multi-material structures targeting orthopedic applications. J Mech Behav Biomed Mater. 2019;94:193-200. [192] PRASAD K, BAZAKA O, CHUA M, et al. Metallic Biomaterials: Current Challenges and Opportunities. Mater Basel Switz. 2017;10(8):884. [193] 马飞,李想,谢瑞敏,等.钙磷涂层镁合金支架的抗腐蚀性及生物相容性[J].中国组织工程研究,2018,22(26):4184-4190. [194] 黄艺轩,杜斌,刘锌,等.聚乙烯亚胺在骨组织工程中的研究进展和应用前景[J].中国组织工程研究,2023,27(16):2563-2570. [195] 刘岩,郑雪新.3D打印聚乳酸-纳米羟基磷灰石/壳聚糖/多西环素抗菌支架的性能[J].中国组织工程研究,2024, 28(22):3532-3538. [196] 王杰杰,殷俊飞扬,钟静,等.3D打印高密度聚乙烯支架表面涂层的性能[J].中国组织工程研究,2023,27(16):2501-2509. [197] 宋美玲,李征宇,艾子政,等.不同比例羟基磷灰石/β-磷酸三钙涂层支架修复骨缺损[J].中国组织工程研究,2023, 27(30):4809-4816. [198] VAN HENGEL IAJ, RIOOL M, FRATILA-APACHITEI LE, et al. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials. 2017; 140:1-15. [199] GLEASON A, BUSH AI. Iron and Ferroptosis as Therapeutic Targets in Alzheimer’s Disease. Neurother J Am Soc Exp Neurother. 2021;18(1):252-264. [200] CHEN LL, FAN YG, ZHAO LX, et al. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorganic Chem. 2023; 131:106301. [201] SQUITTI R, REALE G, TONDOLO V, et al. Imbalance of Essential Metals in Traumatic Brain Injury and Its Possible Link with Disorders of Consciousness. Int J Mol Sci. 2023;24(7): 6867. [202] BJØRKLUND G, MUTTER J, AASETH J. Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch Toxicol. 2017;91(12): 3787-3797. [203] SALL ML, DIAW AKD, GNINGUE-SALL D, et al. Toxic heavy metals: impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ Sci Pollut Res Int. 2020;27(24):29927-29942. [204] 王赛楠,王晓菲,张莉.甲基丙烯酰明胶水凝胶作为细胞三维培养支架在骨组织工程中的应用[J].中国组织工程研究, 2024,28(22):3576-3582. |
| [1] | 刘安婷, 陆江涛, 张文杰, 贺 玲, 唐宗生, 陈晓玲. 血小板裂解物调控腺苷酸活化蛋白激酶抑制镉诱导的神经细胞凋亡[J]. 中国组织工程研究, 2026, 30(7): 1800-1807. |
| [2] | 张天蔚, 韩兴元, 张佃明, 李荣华, 赵德伟. 基于回归分析的生物可降解锌合金接骨板结构设计和有限元分析[J]. 中国组织工程研究, 2026, 30(14): 3485-3493. |
| [3] | 陈伊琳, 蒋晓波, 屈红林, 刘瑞莲. GSK3/Nrf2调控的生物节律在机体衰老中的规律[J]. 中国组织工程研究, 2025, 29(6): 1257-1264. |
| [4] | 田岳凤, 熊罗节, 王慧芳, 翟春涛, 李 玮. 隔药饼灸调节大鼠免疫抑制机制的转录组测序分析[J]. 中国组织工程研究, 2025, 29(5): 978-988. |
| [5] | 刘浩洋, 谢 强, 沈梦然, 任岩松, 马金辉, 王佰亮, 岳德波, 王卫国. 可降解锌基合金在骨缺损修复重建中的应用及研究热点和不足[J]. 中国组织工程研究, 2025, 29(4): 839-845. |
| [6] | 陈耀东, 任家仪, 曹静玮, 樊文文, 陈 武. 近红外光响应性纳米颗粒h-PCuNF介导多模态疗法治疗恶性肿瘤[J]. 中国组织工程研究, 2025, 29(4): 780-788. |
| [7] | 赵 帅, 李冬瑶, 魏岁艳, 曹怡静, 许 燕, 徐国强. 静电纺丝聚偏氟乙烯压电仿生骨膜的生物相容性评价[J]. 中国组织工程研究, 2025, 29(4): 730-737. |
| [8] | 陈 莹, 郭晓婧, 莫雪妮, 马 威, 武尚志, 李相玲, 谢婷婷. 缺血性脑卒中诊断性生物标志物解析及靶向铜死亡相关基因的实验验证[J]. 中国组织工程研究, 2025, 29(35): 7562-7570. |
| [9] | 曾 玉, 谢成伟, 洪苑琪, 苏盛辉, 董谢平. 含铜介孔生物活性玻璃的体外成血管及成骨性能[J]. 中国组织工程研究, 2025, 29(28): 5941-5949. |
| [10] | 程新奇, 邵龙辉, 沈华侨, 刘宏伟. 铜锶二元掺杂硅酸钙涂层改性钛合金的促成骨和抗菌效应[J]. 中国组织工程研究, 2025, 29(22): 4639-4646. |
| [11] | 高红丽, 秦玉凤, 张玥晗, 舒佳玉, 陈河林. 铜代谢与口腔疾病的诊断及治疗[J]. 中国组织工程研究, 2025, 29(20): 4316-4324. |
| [12] | 刘丹丹, 秦合伟. 线粒体自噬、铁死亡、铜死亡和双硫死亡在阿尔茨海默病中的作用机制及进展[J]. 中国组织工程研究, 2025, 29(19): 4132-4144. |
| [13] | 陈桂琳, 汤其强. 阿尔茨海默病NK细胞与铜死亡的相关基因分析[J]. 中国组织工程研究, 2025, 29(19): 4172-4180. |
| [14] | 罗云彩, 孟茂花, 李 英, 王 欢, 陆 婧, 舒佳玉, 李文杰, 孙金熠, 董 强, . 铜元素影响糖尿病并发症的发生与发展[J]. 中国组织工程研究, 2025, 29(17): 3641-3649. |
| [15] | 官振菊, 谢永林, 向守刚, 张成栋, 李小龙, 李兴平, 蒲 超, 张 波, 罗栩伟, 肖东琴. 钛表面多酚介导铜离子涂层制备及抗菌抗氧化性能[J]. 中国组织工程研究, 2025, 29(10): 1997-2005. |
多项实验研究已揭示了某些金属暴露增加(如锰、铁、锌、铜、镉、铅、铝)与神经退行性疾病发生之间的潜在关联[17-23],详细描述见表1。因此,该文阐述金属暴露对神经退行性疾病的影响以及它们对神经退行性疾病作用的神经毒理学机制,见图1;同时,该文还引申出金属毒性研究对神经组织工程的重要启示,具体包括:分析金属污染物对含金属生物材料等组织工程支架的潜在风险,评估金属螯合剂在神经再生疗法中的协同应用潜力,进而有针对性地提出干预金属毒性的组织工程策略。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 由第一作者在2025年2月进行检索。
1.1.2 文献检索时限 各数据库建库至2025年2月。
1.1.3 检索数据库 中国知网、Web of Science和PubMed数据库。
1.1.4 检索词 英文检索词为“Metal exposure,Manganese,Iron,Zinc,Copper,Cadmium,Lead,Aluminum,Neurodegenerative diseases,Alzheimer’s disease,Parkinson‘s disease,Multiple sclerosis,Amyotrophic lateral sclerosis,Huntington’s disease”等,中文检索词为“金属暴露,锰,铁,锌,铜,镉,铅,铝,神经退行性疾病,阿尔茨海默病,帕金森病,多发性硬化,肌萎缩侧索硬化,亨廷顿舞蹈病”等。
1.1.5 检索文献类型 综述、基础研究及临床研究。
1.1.6 检索策略 以PubMed数据库检索策略为例,见图2。
1.1.7 检索文献量 初步检索到5 898 篇文献。
1.2 入组标准
纳入标准:①有关金属暴露在神经退行性疾病中的综述、实验性或基础研究;②相同领域中论点、论据可靠且在权威杂志上发表的文献。
排除标准:①重复性研究及陈旧性文献;②文献资料无法获取的部分文献。
1.3 文献质量评估及数据的提取 对于检索到的5 898篇文献,通过阅读题目及摘要进行初步筛选,排除与文章内容不相关的文献,经全文阅读后筛选出与题目相关的内容,主要为综述、基础研究及临床研究等,最终纳入204篇文献,其中英文文献196篇、中文文献8篇。文献筛选流程图见
图3。
Discussion and conclusion
3.1 既往他人在该领域研究的贡献和存在的问题 随着全球神经退行性疾病发病率持续攀升与环境污染问题日益严峻,金属暴露与神经退行性疾病的关联性研究受到广泛关注。既往研究已取得3方面重要进展:首先,基于广地域、长时间跨度和多人群的流行病学调查,明确了锰、铁、锌、铜、镉、铅、铝等金属的神经毒性,不仅揭示了金属暴露作为环境风险因素在神经退行性疾病中的潜在危害,更为未来研究提供了关键方向指引;其次,从分子机制层面深入解析了金属暴露诱发神经退行性病变的核心病理途径,包括氧化应激爆发、神经炎症级联反应、金属离子稳态失衡及线粒体功能障碍;最后,基于上述机制探索出部分临床转化成果,如金属螯合剂的应用。
然而,当前研究仍存在显著局限:一方面,尽管揭示了金属暴露的潜在影响与作用机制,但金属离子如何通过调控神经炎症、激活氧化应激、扰乱金属稳态或干扰神经细胞代谢等具体生物学途径与疾病发病机制直接关联,尚缺乏充分的实验证据和深层机制阐释;另一方面,多数机制研究集中于单一金属的体外模型,未能充分模拟真实环境中多金属共暴露的复杂交互效应;此外,现有临床转化成果(如金属螯合剂疗法)的疗效仍需大规模验证,而神经组织工程领域虽已提出干预策略,其安全性、效率及临床转化潜力也亟待系统优化,以推动该技术向更可靠、更有效的临床应用迈进。
3.2 该综述区别于他人他篇的特点 尽管已有大量研究探讨金属暴露与神经退行性疾病的关系,但既往综述多聚焦于单方面阐述各类金属暴露的作用机制,对金属如何综合影响此类疾病的发生与发展,尚缺乏系统完整的总结。该文不仅系统梳理了单一金属暴露引发神经毒性的最新研究进展及分子机制,更着重指出:金属暴露造成的神经损伤远非直接的毒性作用,其核心在于激活细胞内外的多重信号通路网络(涉及氧化应激、金属稳态失衡、线粒体损伤等),逐步加剧神经退行性病变,最终导致认知功能衰退与运动功能障碍。此外,该文还探讨了混合金属暴露可能产生的协同或拮抗效应,并引申出金属毒性研究对神经组织工程的重要启示,包括分析金属污染物对含金属生物材料支架的潜在风险、评估金属螯合剂在神经再生疗法中的协同应用潜力,进而提出干预金属毒性的针对性组织工程策略。
3.3 该综述的局限性 由于篇幅所限,未涵盖其他金属的作用及对金属暴露干预措施的深入探讨;受现有文献质量限制,部分金属的神经毒性机制讨论深度不足;未能系统归纳靶向调控金属毒性机制以改善神经退行性疾病的药物治疗策略;纳入的临床研究证据偏重于欧美人群,可能限制结论的普适性;针对金属暴露关键时间窗口(如产前或老年期)的差异性分析亦未充分展开。
3.4 该综述的意义 该文系统整合了锰、铁、锌、铜、镉、铅、铝等多种环境金属暴露与神经退行性疾病关联的关键证据,突破了以往单一金属或单一疾病研究的局限。通过从分子、亚细胞到组织层面的深入解析,揭示了“氧化应激-线粒体损伤-神经炎症-蛋白稳态失衡”这一跨疾病共性机制的核心框架,这一突破不仅填补了多金属协同毒性机制的理论空白,为工业化背景下的环境污染防控与高危职业人群保护提供了直接的科学依据,更关键的是深化了对金属神经毒性复杂性的理解,超越了简单的直接毒性认知。此外,研究前瞻性地探讨了混合金属暴露可能产生的协同或拮抗复合效应,为开发金属螯合剂-抗氧化-抗炎联合疗法开辟了新的干预路径。尤为重要的是,该文系统地将金属毒性研究的视角拓展至神经组织工程领域,深入剖析了含金属生物材料作为支架的潜在风险,评估了金属螯合剂在神经再生疗法中的协同应用潜力,并最终提出了基于智能递送系统和响应性材料等前沿技术的针对性组织工程干预策略。通过深入挖掘金属特异性靶点(如靶向NLRP3抑制铝诱导的细胞焦亡、调控Rab27a阻断锰介导的有害外泌体传播),该文为开发新型药物和精准疗法提供了重要的新思路。综上所述,这一系列发现不仅为深刻理解神经退行性疾病的金属致病机制提供了更全面深入的见解,更在实践层面为开发基于组织工程原理、主动干预金属毒性以促进神经修复与再生的创新疗法奠定了理论基础,并指明了未来极具前景的研究方向。
3.5 课题专家组对未来的建议 课题专家组经深入研讨达成共识,建议未来研究需重点突破以下关键方向:在机制层面,亟需通过整合高通量测序、多组学分析与跨尺度模型(动物-临床),系统解析金属暴露诱发神经退行性病变的动态过程,从细胞代谢紊乱、神经环路异常到器官功能障碍,填补金属离子介导的神经炎症激活、氧化应激爆发及金属稳态失衡等通路与疾病发病机制间的直接证据缺口;在环境复杂性层面,应着力探究真实场景中多金属共暴露的协同/拮抗效应及其与遗传背景的交互作用,这不仅是阐明疾病异质性和个体易感差异的核心,更是制定精准预防策略的科学基石;在临床转化层面,随着金属螯合剂等药物的革新,未来需开发兼具金属识别特异性、代谢调控能力及低不良反应的智能治疗策略(如响应性纳米载体、工程化生物材料),同时加速神经组织工程干预方案的临床验证与优化,以实现神经毒性的靶向清除与神经再生微环境的主动调控和修复。
综上所述,金属暴露与神经退行性疾病的关联研究仍存在重大探索空间,未来工作不仅需纵深破解金属致病的分子网络与环境-基因互作机制,更需推动研究成果向临床落地转化,最终构建覆盖早期生物标志物筛查、高危人群防护与个体化治疗的全链条防控体系。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||