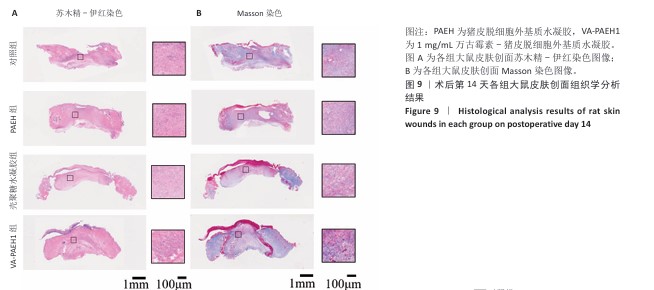
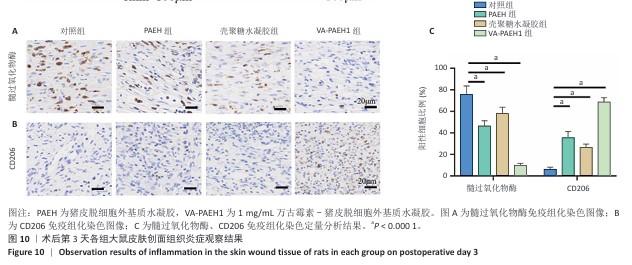
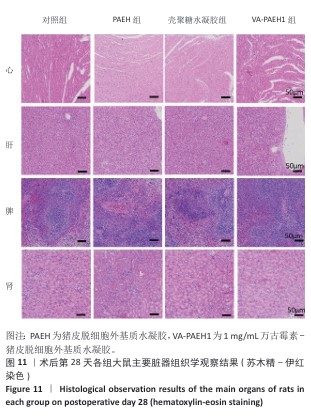
[1] LI Q, SONG H, LI S, et al. Macrophage metabolism reprogramming EGCG-Cu coordination capsules delivered in polyzwitterionic hydrogel for burn wound healing and regeneration. Bioact Mater. 2023;29:251-264.
[2] WANG Y, BEEKMAN J, HEW J, et al. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3-17.
[3] SINGER AJ, CLARK RA. Cutaneous wound healing. N Engl J Med. 1999; 341(10):738-746.
[4] QIAN Y, ZHENG Y, JIN J, et al. Immunoregulation in Diabetic Wound Repair with a Photoenhanced Glycyrrhizic Acid Hydrogel Scaffold. Adv Mater. 2022;34(29):e2200521.
[5] 李小鸾.10%聚维酮碘乳膏治疗皮肤感染性创面的疗效观察[J]. 中国实用医药,2020,15(13):143-145.
[6] 王廷宇,叶子青,郭晓雨,等.夫西地酸治疗浅II度烧伤的疗效及对烧伤常见菌的体外抗菌活性探讨[J].中国美容医学,2023, 32(4):34-37.
[7] BLACKLOW SO, LI J, FREEDMAN BR, et al. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci Adv. 2019; 5(7):eaaw3963.
[8] ZHAO X, WU H, GUO B, et al. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34-47.
[9] YANG Z, HUANG R, ZHENG B, et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv Sci (Weinh). 2021;8(8):2003627.
[10] UBEROI A, MCCREADY-VANGI A, GRICE EA. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat Rev Microbiol. 2024;22(8):507-521.
[11] NOVIELLO S, COREY GR, HOLLAND TL, et al. A pooled analysis of patients with wound infections in the Phase 3 REVIVE trials: randomized, double-blind studies to evaluate the safety and efficacy of iclaprim versus vancomycin for treatment of acute bacterial skin and skin structure infections. J Med Microbiol. 2020;69(4):625-630.
[12] 常欣悦,徐丽,吴晓旭.急性细菌性皮肤感染病原菌耐药性及万古霉素治疗效果[J].中华医院感染学杂志,2022,32(5):745-749.
[13] CHEN Y, WANG X, TAO S, et al. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil Med Res. 2023;10(1):37.
[14] ZHANG W, WU W, WANG T, et al. Novel Supramolecular Hydrogel for Infected Diabetic Foot Ulcer Treatment. Adv Healthc Mater. 2024; 13(31):e2402092.
[15] KOWALEWSKI M, PASIERSKI M, MAKHOUL M, et al. Topical vancomycin for sternal wound infection prophylaxis. A systematic review and updated meta-analysis of over 40,000 cardiac surgery patients. Surgery. 2023;174(5):1102-1112.
[16] 刘旭良,李伯庭,郑介柏,等.负压封闭引流技术联合外用万古霉素治疗骶尾部难愈性压疮的效果[J].医学信息,2022,35(17): 37-40.
[17] 何家伟,王菁,汪洋,等.局部应用万古霉素粉末预防膝和髋关节置换术后手术部位感染有效性和安全性的meta分析[J].中国现代应用药学,2024,41(6):812-822.
[18] SALEH A, THABET A, BELKHAIR S. Topical Vancomycin for Prevention of Surgical Site Infection after Craniotomy: Meta-analysis and Systematic Literature Review. World Neurosurg. 2022;158:e605-e611.
[19] MA H, SIU WS, LEUNG PC. The Potential of MSC-Based Cell-Free Therapy in Wound Healing-A Thorough Literature Review. Int J Mol Sci. 2023;24(11):9356.
[20] 石晶,楚瑨,孙涛,等.脱细胞基质-甲基丙烯酰化明胶复合水凝胶的制备及其对肝细胞增殖的作用[J].国际生物医学工程杂志, 2025,48(1):47-55.
[21] SRICHAIYAPOL O, MADDOCKS SE, THAMMAWITHAN S, et al. TA-AgNPs/Alginate Hydrogel and Its Potential Application as a Promising Antibiofilm Material against Polymicrobial Wound Biofilms Using a Unique Biofilm Flow Model. Microorganisms. 2022;10(11):2279.
[22] JIN F, LI X, CHEN J, et al. Clinical study on the role of platelet-rich plasma in human acellular dermal matrix with razor autologous skin graft repair of giant congenital pigmented nevus in children. J Plast Reconstr Aesthet Surg. 2024;90:305-314.
[23] TAMAYO L, SANTANA P, FORERO JC, et al. Coaxial fibers of poly(styrene-co-maleic anhydride)@poly(vinyl alcohol) for wound dressing applications: Dual and sustained delivery of bioactive agents promoting fibroblast proliferation with reduced cell adherence. Int J Pharm. 2022;611:121292.
[24] DONG Q, ZU D, KONG L, et al. Construction of antibacterial nano-silver embedded bioactive hydrogel to repair infectious skin defects. Biomater Res. 2022;26(1):36.
[25] KONG P, DONG J, LI W, et al. Extracellular Matrix/Glycopeptide Hybrid Hydrogel as an Immunomodulatory Niche for Endogenous Cardiac Repair after Myocardial Infarction. Adv Sci (Weinh). 2023; 10(23):e2301244.
[26] IYER V, RAUT J, DASGUPTA A. Impact of pH on growth of Staphylococcus epidermidis and Staphylococcus aureus in vitro. J Med Microbiol. 2021;70(9).doi:10.1099/jmm.0.001421.
[27] MO GY, ZHANG SC, LI YX, et al. [Establish mouse osteoblast -osteoclast cell co-culture system in a Transwell chamber]. Zhongguo Gu Shang. 2018;31(3):241-247.
[28] LI Y, WANG Y, DING Y, et al. A Double Network Composite Hydrogel with Self-Regulating Cu2+/Luteolin Release and Mechanical Modulation for Enhanced Wound Healing [published correction appears in ACS Nano. 2024 Dec 17;18(50):34415-34418.
[29] SIM HW, LEE WY, LEE R, et al. The Anti-Inflammatory Effects of Broccoli (Brassica oleracea L. var. italica) Sprout Extract in RAW 264.7 Macrophages and a Lipopolysaccharide-Induced Liver Injury Model. Curr Issues Mol Biol. 2023;45(11):9117-9131.
[30] CAI D, CHEN S, WU B, et al. Construction of multifunctional porcine acellular dermal matrix hydrogel blended with vancomycin for hemorrhage control, antibacterial action, and tissue repair in infected trauma wounds. Mater Today Bio. 2021;12:100127.
[31] FANG B, QIU P, XIA C, et al. Extracellular matrix scaffold crosslinked with vancomycin for multifunctional antibacterial bone infection therapy. Biomaterials. 2021;268:120603.
[32] THITIANANPAKORN K, AIBA Y, TAN XE, et al. Association of mprF mutations with cross-resistance to daptomycin and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA). Sci Rep. 2020; 10(1):16107.
[33] LI F, LIU T, LIU X, et al. Ganoderma lucidum polysaccharide hydrogel accelerates diabetic wound healing by regulating macrophage polarization. Int J Biol Macromol. 2024;260(Pt 2):129682.
[34] LEE J, BYUN H, MADHURAKKAT PERIKAMANA SK, et al. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv Healthc Mater. 2019;8(4):e1801106.
[35] WANG Y, GAO H, WANG X, et al. Dual cross-linked self-healing hydrogel enhanced by dopamine nanoparticles and raffinose for wound healing. Int J Biol Macromol. 2024;271(Pt 1):132615.
[36] YAO J, XIAO L, LIN MJ, et al. Bifunctional copper ion/melittin incorporated hydrogel with antimicrobial and antioxidant capabilities for infected skin wound healing. Mater Design. 2025;250:113631.
[37] XU Y, HU Q, WEI Z, et al. Advanced polymer hydrogels that promote diabetic ulcer healing: mechanisms, classifications, and medical applications. Biomater Res. 2023;27(1):36.
[38] GE Y, RONG F, LU Y, et al. Glucose Oxidase Driven Hydrogen Sulfide-Releasing Nanocascade for Diabetic Infection Treatment. Nano Lett. 2023;23(14):6610-6618.
[39] LIU S, HUANG Y, JENSEN S, et al. Molecular physiological characterization of the dynamics of persister formation in Staphylococcus aureus. Antimicrob Agents Chemother. 2024;68(1):e0085023.
[40] SUN S, WANG Q, ZHANG B, et al. Vancomycin-Loaded in situ Gelled Hydrogel as an Antibacterial System for Enhancing Repair of Infected Bone Defects. Int J Nanomedicine. 2024;19:10227-10245.
[41] ANDRIANOPOULOU A, SOKOLOWSKI K, WENZLER E, et al. Assessment of antibiotic release and antibacterial efficacy from pendant glutathione hydrogels using ex vivo porcine skin. J Control Release. 2024;365:936-949.
[42] LIU W, GAO R, YANG C, et al. ECM-mimetic immunomodulatory hydrogel for methicillin-resistant Staphylococcus aureus-infected chronic skin wound healing. Sci Adv. 2022;8(27):eabn7006.
[43] DEURENBERG RH, STOBBERINGH EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8(6):747-763.
[44] WALTERS MS, EGGERS P, ALBRECHT V, et al. Vancomycin-Resistant Staphylococcus aureus - Delaware, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(37):1056.
[45] LI D, LI J, XU Y, et al. Topical vancomycin powder for the prevention of surgical site infections in spinal deformity surgery: a systematic review and meta-analysis. Eur Spine J. 2024;33(12):4653-4663.
[46] GHOSH S, MUKHERJEE S, PATRA D, et al. Polymeric Biomaterials for Prevention and Therapeutic Intervention of Microbial Infections. Biomacromolecules. 2022;23(3):592-608.
[47] Gao X, Chai X, Lou Y, et al. Fabrication of porcine acellular dermal matrix and oxidized hyaluronic acid conductive composite hydrogels for accelerating skin wound healing under electrical stimulation. Int J Biol Macromol. 2024;282(Pt 4):137179.
[48] CANNON JGD, HO AL, MOHOLE J, et al. Topical vancomycin for surgical prophylaxis in non-instrumented pediatric spinal surgeries. Childs Nerv Syst. 2019;35(1):107-111.
[49] FURNARY AP, WU Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract. 2006;12 Suppl 3:22-26.
[50] LAZAR HL, KETCHEDJIAN A, HAIME M, et al. Topical vancomycin in combination with perioperative antibiotics and tight glycemic control helps to eliminate sternal wound infections. J Thorac Cardiovasc Surg. 2014;148:1035-1038;1038-1040.
|