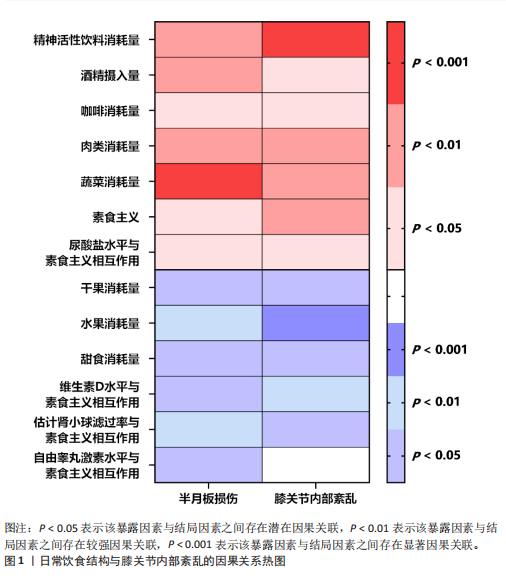
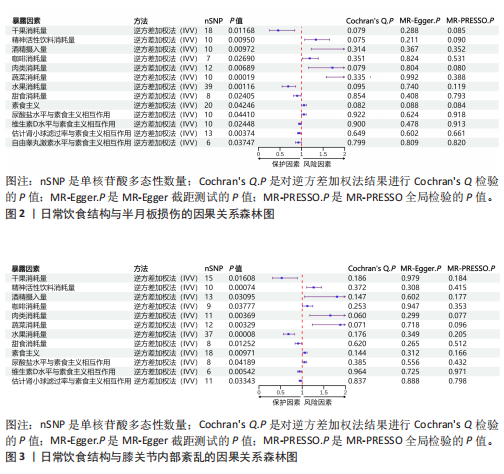
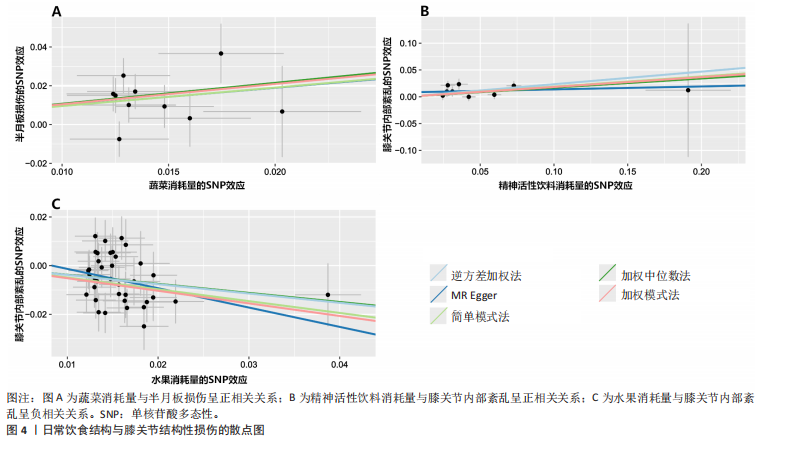
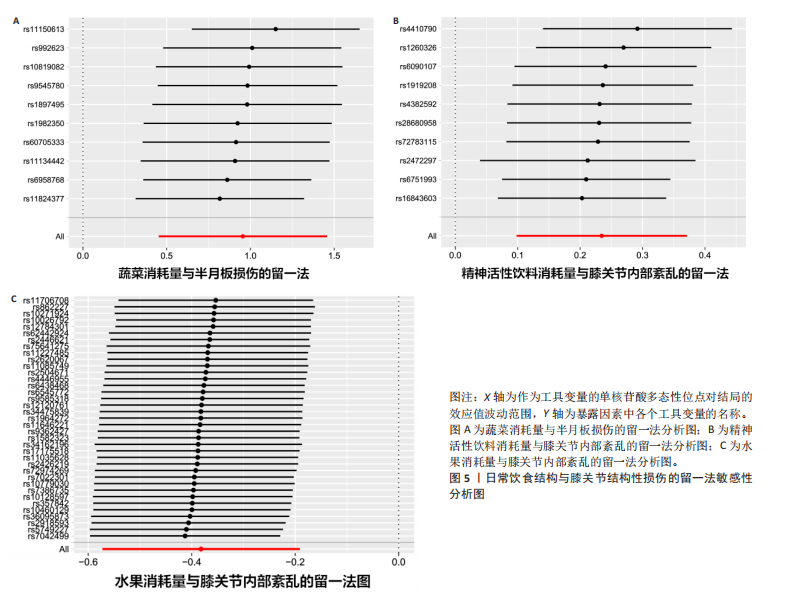
[1] MEZHOV V, TEICHTAHL AJ, STRASSER R, et al. Meniscal pathology - the evidence for treatment. Arthritis Res Ther. 2014;16(2): 206.
[2] PALMOWSKI Y, JUNG T, DOERING AK, et al. Analysis of cartilage injury patterns and risk factors for knee joint damage in patients with primary lateral patella dislocations. PLoS One. 2021;16(10):e0258240.
[3] HEUTS EAF, DE JONG LD, HAZEBROEK EJ, et al. The influence of bariatric surgery on hip and knee joint pain: a systematic review. Surg Obes Relat Dis. 2021;17(9):1637-1653.
[4] YAMAMOTO Y, MURATA Y, TANAKA N, et al. Mobile application for home exercise adherence in patients with knee osteoarthritis: A pilot study. Medicine (Baltimore). 2022;101(42):e31181.
[5] DUONG V, OO WM, DING C, et al. Evaluation and Treatment of Knee Pain: A Review. JAMA. 2023;330(16):1568-1580.
[6] HUFFMAN KF, AMBROSE KR, NELSON AE, et al. The Critical Role of Physical Activity and Weight Management in Knee and Hip Osteoarthritis: A Narrative Review. J Rheumatol. 2024;51(3):224-233.
[7] IDOWU BM, AFOLABI BI, ONIGBINDE SO, et al. Magnetic Resonance Imaging of Internal Derangements and Other Knee Pathologies in Adult Nigerians. Niger Med J. 2023;64(4):569-581.
[8] MA C, SEARLE D, TIAN J, et al. Dietary Inflammatory Index and Magnetic Resonance Imaging-Detected Knee Structural Change and Pain: A 10.7-Year Follow-up Study. Arthritis Care Res (Hoboken). 2024;76(6):813-820.
[9] EMDIN CA, KHERA AV, KATHIRESAN S. Mendelian Randomization. JAMA. 2017; 318(19):1925-1926.
[10] LAWLOR DA, HARBORD RM, STERNE JA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163.
[11] SPIGA F, GIBSON M, DAWSON S, et al. Tools for assessing quality and risk of bias in Mendelian randomization studies: a systematic review. Int J Epidemiol. 2023; 52(1):227-249.
[12] DIDELEZ V, SHEEHAN N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16(4):309-330.
[13] KURKI MI, KARJALAINEN J, PALTA P, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508-518.
[14] 王晶,张国燕,程杉孟德尔随机化的良好实践:孟德尔随机化分析的常见设计、关键挑战及优化[J].首都医科大学学报, 2023,44(6):1087-1094.
[15] SANNA S, VAN ZUYDAM NR, MAHAJAN A, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019; 51(4):600-605.
[16] BURGESS S, BUTTERWORTH A, THOMPSON SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658-665.
[17] XIANG K, WANG P, XU Z, et al. Causal Effects of Gut Microbiome on Systemic Lupus Erythematosus: A Two-Sample Mendelian Randomization Study. Front Immunol. 2021; 12:667097.
[18] BOWDEN J, DAVEY SMITH G, et al. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525.
[19] BOWDEN J, DAVEY SMITH G, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304-314.
[20] GRECO M FD, MINELLI C, SHEEHAN NA, et al. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015; 34(21):2926-2940.
[21] BURGESS S, DAVEY SMITH G, DAVIES NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2023;4:186.
[22] PETROSKI W, MINICH DM. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020;12(10):2929.
[23] AKTER S, NETZEL M, TINGGI U, et al. Interactions Between Phytochemicals and Minerals in Terminalia ferdinandiana and Implications for Mineral Bioavailability. Front Nutr. 2020;7:598219.
[24] ZHANG YY, STOCKMANN R, NG K, et al. Revisiting phytate-element interactions: implications for iron, zinc and calcium bioavailability, with emphasis on legumes. Crit Rev Food Sci Nutr. 2022;62(6):1696-1712.
[25] TERRELL K, CHOI S, CHOI S. Calcium’s Role and Signaling in Aging Muscle, Cellular Senescence, and Mineral Interactions. Int J Mol Sci. 2023;24(23):17034.
[26] LI M, NIE Y, LI K, et al. Effect of Extensor Muscle Strength on Meniscus Damage Progression in Subjects Without Radiologic Knee Osteoarthritis: Data From the Osteoarthritis Initiative. Am J Phys Med Rehabil. 2022;101(9):836-842.
[27] COSTA MI, SARMENTO-RIBEIRO AB, GONÇALVES AC. Zinc: From Biological Functions to Therapeutic Potential. Int J Mol Sci. 2023;24(5):4822.
[28] LI Q, WEN Y, WANG L, et al. Hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Exp Mol Med. 2021; 53(11):1735-1747.
[29] GOULDIN AG, PATEL NK, GOLLADAY GJ, et al. Advanced glycation end-product accumulation differs by location and sex in aged osteoarthritic human menisci. Osteoarthritis Cartilage. 2023;31(3):363-373.
[30] BERMAN NK, HONIG S, CRONSTEIN BN, et al. The effects of caffeine on bone mineral density and fracture risk. Osteoporos Int. 2022;33(6):1235-1241.
[31] CHEN B, HE Q, CHEN C, et al. Combination of curcumin and catalase protects against chondrocyte injury and knee osteoarthritis progression by suppressing oxidative stress. Biomed Pharmacother. 2023;168:115751.
[32] NEJADHOSSEINIAN M, DJALALINIA S, HAERIAN H, et al. The effects of antioxidants on knee osteoarthritis: A systematic review and meta-analysis. Front Nutr. 2022;9: 1026450.
[33] LU W, SHI Y, WANG R, et al. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int J Mol Sci. 2021;22(9):4945.
[34] THALER R, KHANI F, STURMLECHNER I, et al. Vitamin C epigenetically controls osteogenesis and bone mineralization. Nat Commun. 2022;13(1):5883.
[35] LIS DM, JORDAN M, LIPUMA T, et al. Collagen and Vitamin C Supplementation Increases Lower Limb Rate of Force Development. Int J Sport Nutr Exerc Metab. 2022;32(2):65-73.
[36] GO DJ, KIM DH, KIM JY, et al. Serum uric acid and knee osteoarthritis in community residents without gout: a longitudinal study. Rheumatology (Oxford). 2021;60(10):4581-4590.
[37] WANG R, WANG ZM, XIANG SC, et al. Relationship between 25-hydroxy vitamin D and knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front Med (Lausanne). 2023;10:1200592.
[38] JOSEPH GB, MCCULLOCH CE, NEVITT MC, et al. Associations Between Vitamins C and D Intake and Cartilage Composition and Knee Joint Morphology Over 4 Years: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2020;72(9):1239-1247.
[39] PARK TY, CHOI MY, KONG D, et al. Do Strength and Anthropometric Size of the Lower Body Correlate with Serum Testosterone Levels? World J Mens Health. 2025;43(1):205-212.
[40] MANZO-AVALOS S, SAAVEDRA-MOLINA A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7(12):4281-4304.
[41] ZHU D, WANG X, XI Z, et al. Diet influences knee osteoarthritis osteophyte formation via gut microbiota and serum metabolites. iScience. 2024;27(6):110111.
[42] TURNBULL J, JHA RR, BARRETT DA, et al. The Effect of Acute Knee Injuries and Related Knee Surgery on Serum Levels of Pro- and Anti-inflammatory Lipid Mediators and Their Associations With Knee Symptoms. Am J Sports Med. 2024;52(4):987-997.
[43] FORTIER LA, BARKER JU, STRAUSS EJ, et al. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715.
|