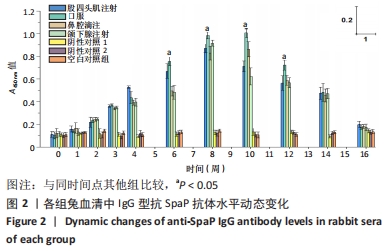
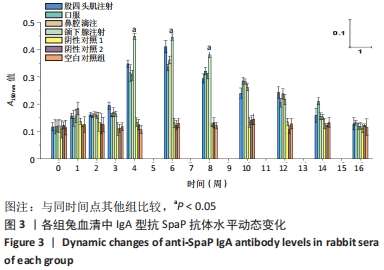
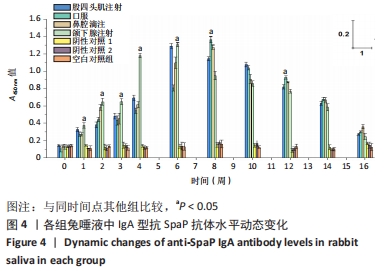
[1] MATSUMOTO-NAKANO M, FUJITA K, OOSHIMA T. Comparison of glucan-binding proteins in cariogenicity of Streptococcus mutans. Oral Microbiol Immunol. 2007;22(1):30-35.
[2] 张睿,樊明文.DNA防龋疫苗不同途径免疫BALB/c小鼠的实验研究[J].牙体牙髓牙周病学杂志,2003,13(5):270-272.
[3] 关薇薇,顾瑜,管晓燕,等.表达嵌合蛋白PAcA/CTB转基因番茄疫苗可通过胃肠道吸收的方式免疫大鼠[J].中国组织工程研究, 2021,25(11):1712-1716.
[4] 亓鹏.温敏型生物降解水凝胶载体防龋基因疫苗不同途径免疫SD大鼠的实验研究[D].遵义:遵义医学院,2011.
[5] 白国辉,刘建国,柴巧学,等.变异链球菌表面蛋白SpaP/P区真核表达质粒的构建及表达[J].中国组织工程研究与临床康复,2010, 14(15):2765-2768.
[6] JIN LJ, LMSTER IB, GREENSPAN JS, et al. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22(7):609-619.
[7] NOMURA R, MATAYOSHI S, OTSUGU M, et al. Contribution of Severe Dental Caries Induced by Streptococcus mutans to the Pathogenicity of Infective Endocarditis. Infect Immun. 2020;88(7):e00897-19.
[8] JAVED S, ZAKIRULLA M, BAIG RU, et al. Development of artificial neural network model for prediction of post-streptococcus mutans in dental caries. Comput Methods Programs Biomed. 2020;186:105198.
[9] SOUNAH SA, MADFA AA. Correlation between dental caries experience and the level of Streptococcus mutans and lactobacilli in saliva and carious teeth in a Yemeni adult population. BMC Res Notes. 2020;13(1): 112.
[10] GROSS EL, BEALL CJ, KUTSCH SR, et al. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 2012;7(10):e47722.
[11] WU Z, GONG Y, WANG C, et al. Association between salivary s-IgA concentration and dental caries: A systematic review and meta-analysis. Biosci Rep. 2020;40(12):BSR20203208.
[12] LETIERI ADS, FREITAS-FERNANDES LB, VALENTE APC, et al. Longitudinal Evaluation of Salivary Iga-S in Children with Early Childhood Caries Before and After Restorative Treatment. J Clin Pediatr Dent. 2019;43(4): 239-243.
[13] SMITH DJ, MATTOS-GRANER RO. Secretory immunity following mutans streptococcal infection or immunization. Curr Top Microbiol Immunol. 2008;319(1):131-156.
[14] CAO XX, FAN J, CHEN J, et al. Immunogenicity and Prediction of Epitopic Region of Antigen AgⅠ/Ⅱ and Glucosyltransferase from Streptococcus mutans. J Huazhong Univ Sci Technolog Med Sci. 2016; 36(3):416-421.
[15] WANG K, ZHOU X, LI W, et al. Human salivary proteins and their peptidomimetics: Values of function, early diagnosis, and therapeutic potential in combating dental caries. Arch Oral Biol. 2019;99:31-42.
[16] BATISTA MT, FERREIRA EL, PEREIRA GDS, et al. LT adjuvant modulates epitope specificity and improves the efficacy of murine antibodies elicited by sublingual vaccination with the N-terminal domain of Streptococcus mutans P1. Vaccine. 2017;35(52):7273-7282.
[17] SU LK, YU F, LI ZF, et al. Intranasal co-delivery of IL-6 gene enhances the immunogenicity of anti-caries DNA vaccine. Acta Pharmacol Sin. 2014;35(5):592-598.
[18] 金洁,孙成,李午丽.人参皂苷Re对防龋疫苗rPAc免疫效应的影响[J].安徽医科大学学报,2018,53(7):1062-1067.
[19] CHEN F, WANG D. Novel technologies for the prevention and treatment of dental caries: a patent survey. Expert Opin Ther Pat. 2010;20(5): 681-694.
[20] BATISTA MT, SOUZA RD, FERREIRA EL, et al. Immunogenicity and in vitro and in vivo protective effects of antibodies targeting a recombinant form of the Streptococcus mutans P1 surface protein. Infect Immun. 2014;82(12):4978-4988.
[21] YANG H, YAN Z, ZHANG Z, et al. Anti-caries vaccine based on clinical cold-adapted influenza vaccine: A promising alternative for scientific and public-health protection against dental caries. Med Hypotheses. 2019;126:42-45.
[22] JIANG H, HU Y, YANG M, et al. Enhanced immune response to a dual-promoter anti-caries DNA vaccine orally delivered by attenuated Salmonella typhimurium. Immunobiology. 2017;222(5):730-737.
[23] NOGALES A, MARTÍNEZ-SOBRIDO L. Reverse Genetics Approaches for the Development of Influenza Vaccines. Int J Mol Sci. 2016;18(1):20.
[24] 李锐,李多慧,全大萍,等.新型温敏型肝素-泊洛沙姆水凝胶包载神经生长因子对糖尿病外周神经损伤修复的作用[J].中国药学杂志,2019,54(12):992-999.
[25] REN Q, DING L, LI Z, et al. Chitosan hydrogel containing amelogenin-derived peptide: Inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesion. Arch Oral Biol. 2019;100:42-48.
[26] YU M, YANG Y, ZHU C, et al. Advances in the transepithelial transport of nanoparticles. Drug Discov Today. 2016;21(7):1155-1161.
[27] 管晓燕,李敏,刘建国,等.温敏型生物降解水凝胶载体防龋基因疫苗pVAX1-spap/A经不同途径免疫新西兰大白兔的实验研究[J].口腔医学研究,2014,30(10):934-938.
[28] JIA R, YAN L, GUO J. Enhancing the immunogenicity of a DNA vaccine against Streptococcus mutans by attenuating the inhibition of endogenous miR-9. Vaccine. 2020;38(6):1424-1430.
[29] FLORENCE AT. Nanoparticle uptake by the oral route: Fulfilling its potential? Drug Discov Today Technol. 2005;2(1):75-81.
[30] 朱卫丰,丁权,李文栋,等.口服纳米颗粒在胃肠道中的跨膜转运研究进展[J].中国实验方剂学杂志,2021,27(9):215-223.
[31] YANG J, DENG D, BRANDT BW, et al. Diversity of SpaP in genetic and salivary agglutinin mediated adherence among Streptococcus mutans strains. Sci Rep. 2019;9(1):19943.
|