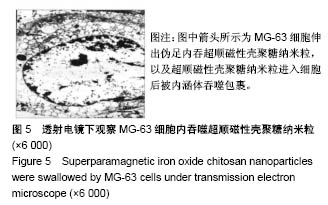
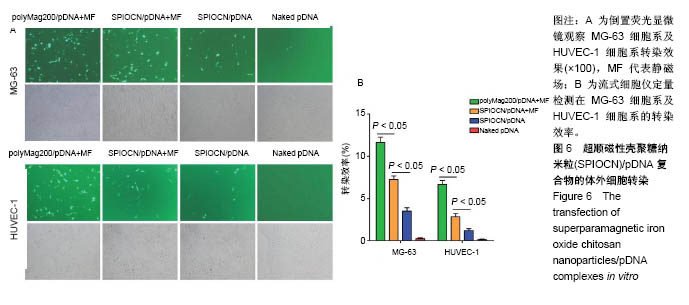
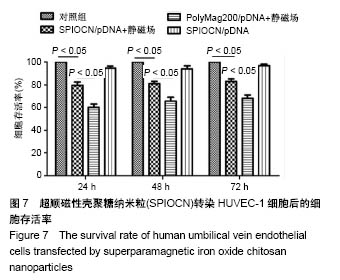
| [1] Duan X,Li W,Xiang Z.Research progress of angiogenesis in vascularized tissue engineered bone.Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.2015,29(2):239-244. [2] Nakano K,Murata K,Omokawa S,et al.Promotion of Osteogenesis and Angiogenesis in Vascularized Tissue-Engineered Bone Using Osteogenic Matrix Cell Sheets.Plast Reconstr Surg. 2016;137(5): 1476-1484.[3] Chen K,Zhang C,Wang L,et al.Progress on strategies to promote vascularization in bone tissue engineering.Zhongguo Gu Shang. 2015;28(4):383-388.[4] Davies N,Dobner S,Bezuidenhout D,et al.The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials.2008;29(26):3531-3538.[5] CasselI OC,Hofer SO,Morrison WA,et al.Vascularisation of tissue-engineered grafts:the regulation of angiogenesis in reconstructive surgery and in disease states.Br J Plast Surg. 2002;55(8):603-610.[6] 金丹,陈滨,裴国献,等.筋膜瓣促组织工程骨再血管化及山羊长段骨缺损的修复[J].中华实验外科杂志,2005,22(3):269-271. [7] Jayaraman P,Gandhimathi C,Venugopal JR,et al.Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering.Adv Drug Deliv Rev.2015,94:77-95.[8] Yin J,Qiu S,Shi B,et al.Controlled release of FGF-2 and BMP-2 in tissue engineered periosteum promotes bone repair in rats. Biomed Mater.2018;13(2):025001.[9] Witlox MA,Lamfers ML,Wuisman PI,et al.Evolving gene therapy approaches for osteosarcoma using viral vectors: review. Bone. 2007;40(4):797-812.[10] Hartono SB,Yu M,Gu W,et al.Synthesis of multi-functional large pore mesoporous silica nanoparticles as gene carriers. Nanotechnology.2014;25(5):055701.[11] Wang Y,Li L,Shao N,et al.Triazine-modified dendrimer for efficient TRAIL gene therapy in osteosarcoma.Acta Biomater. 2015;17: 115-124.[12] Wu P,Chen H,Jin R,et al.Non-viral gene delivery systems for tissue repair and regeneration.J Transl Med.2018;16(1):29.[13] Helal NA,Osami A,Helmy A,et al.Non-viral gene delivery systems: hurdles for bench-to-bedside transformation. Pharmazie. 2017; 72(11):627-693.[14] Ishii T,Okahata Y,Sato T.Mechanism of cell transfection with plasmid/chitosan complexes. Biochim Biophys Acta. 2001; 1514(1):51-64.[15] Vainauska D,Kozireva S,Karpovs A,et al.A novel approach for nucleic acid delivery into cancer cells.Medicina(Kaunas). 2012; 48(6):324-329.[16] Smolders S,Kessels S,Smolders SM,et al.Magnetofection is superior to other chemical transfection methods in a microglial cell line.J Neurosci Methods.2017;293:169-173.[17] Laurentt N,Sapet C,Le GL,et al.Nucleic acid delivery using magnetic nanoparticles: the Magnetofection technology.Ther Deliv.2011;2(4):471-482.[18] Crane GM,Ishaug SL,Mikos AG.Bone tissue engineering.Nat Med.1995;1(12):1322-1324.[19] Carmeliet P.Mechanisms of angiogenesis and arteriogenesis.Nat Med.2000,6(4):389-395.[20] Oda M.Prefabrication of vascularized bone grafts measured with laser Doppler flowmetry: An experimental study in rats.Scand I Plast Reconstr Surg Hand Surg. 1994;28:249-252.[21] Wamke PH,Springer IN,Wiltfang J.Growth and transphantation of a custom vascularised bone graft in a man. Lancet. 2004;364 (9436):766-770. [22] 汪群力,裴国献.组织工程组织血管化研究新进展[J].中华创伤骨科杂志,2005,7(6):564-567.[23] Kasper FK,Melville J,Shum J,et al.Tissue Engineered Prevascularized Bone and Soft Tissue Flaps.Oral Maxillofac Surg Clin North Am.2017;29(1):63-73.[24] Kaempfen A,Todorov A,Güven S,et al.Engraftment of Prevascularized, Tissue Engineered Constructs in a Novel Rabbit Segmental Bone Defect Model.Int J Mol Sci. 2015;16(6): 12616-12630.[25] Nguyen LH,Annabi N,Nikkhah M,et al.Vascularized Bone Tissue Engineering:Approaches for Potential Improvement.Tissue Eng Part B Rev.2012;18(5):363-382.[26] Bussemer T,Dashevsky A,Bodmeier R.A pulsatile drug delivery system based on rupturable coated hard gelatin capsules.J Control Release.2003;93(3):331-933.[27] Solorio L,Zwolinski C,Lund AW,et a1.Gelatin microspheres crosslinked with genipin for local delivery of growth factors.J Tissue Eng Regen Med.2010;4(7):514-523.[28] Jabbarzadeh E,Starnes T,Khan YM,et al.Induction of angiogenesis in tissue-engineered scafolds designed for bone repair:A combined gene therapy-cell transplantation approach.Proc NatI Acad Sci USA. 2008;105(32):11099-11104.[29] Yang W,Wang F,Feng L,et al.Applications and prospects of non-viral vectors in bone regeneration.Curr Gene Ther.2018.doi:10.2174/1566523218666180227154232.[30] Hardee CL,Arévalo-Soliz LM,Hornstein BD,et al.Advances in Non-Viral DNA Vectors for Gene Therapy. Genes(Basel). 2017;8(2).pii:E65.doi:10.3390/genes8020065.[31] Slivac I,Guay D,Mangion M,et al.Non-viral nucleic acid delivery methods.Expert Opin Biol Ther. 2017;17(1):105-118.[32] Long Z,Zhang J,Shen Y,et al.Polyethyleneimine grafted short halloysite nanotubes for gene delivery.Mater Sci Eng C Mater Biol Appl.2017;81:224-235.[33] Del Pozo-Rodríguez A,Solinís MÁ,Rodríguez-Gascón A,et al. Applications of lipid nanoparticles in gene therapy.Eur J Pharm Biopharm.2016;109:184-193.[34] Raftery R,O'Brien FJ,Cryan SA.Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules. 2013; 18(5):5611-5647.[35] Sohrabijam Z,Saeidifar M,Zamanian A,et al.Enhancement of magnetofection efficiency using chitosan coated superparamagnetic iron oxide nanoparticles and calf thymus DNA.Colloids Surf B Biointerfaces.2017;152:169-175.[36] Pan Z,Shi Z,Wei H,et al.Magnetofection based on superparamagnetic iron oxide nanoparticles weakens glioma stem cell proliferation and invasion by mediating high expression of microRNA-374a. J Cancer. 2016;7(11):1487-1496.[37] Wang YX,Xuan S,Port M,et al.Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research.Curr Pharm Des. 2013;19(37): 6575-6593.[38] Wang X,Chen B,Yang X,et al.Functionalized superparamagnetic nanoparticles for highly-efficient gene delivery.J Nanosci Nanotechnol.2013;13(2):746-750.[39] Moghimi SM,Symonds P,Murray JC,et al.A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy.Mol Ther.2005;11(6):990-995. [40] Edelman ER,Kost J,Bobeck H,et al.Regul- ation of drug release from polymer matrices by oscillating magnetic fields.J Biomed Mater Res.1985;19:67-83. |
.jpg)

.jpg)